Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease - PubMed (original) (raw)
Comparative Study
Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease
Takashi Kudo et al. Exp Neurol. 2011 Mar.
Abstract
Many patients with Huntington's disease (HD) exhibit disturbances in their daily cycle of sleep and wake as part of their symptoms. These patients have difficulty sleeping at night and staying awake during the day, which has a profound impact on the quality of life of the patients and their care-givers. In the present study, we examined diurnal and circadian rhythms of four models of HD including the BACHD, CAG 140 knock-in and R6/2 CAG 140 and R6/2 CAG 250 lines of mice. The BACHD and both R6/2 lines showed profound circadian phenotypes as measured by wheel-running activity. Focusing on the BACHD line for further analysis, the amplitude of the rhythms in the BACHD mice declined progressively with age. In addition, the circadian regulation of heart rate and body temperature in freely behaving BACHD mice were also disrupted. Furthermore, the distribution of sleep as well as the autonomic regulation of heart rate was disrupted in this HD model. To better understand the mechanistic underpinnings of the circadian disruption, we used electrophysiological tools to record from neurons within the central clock in the suprachiasmatic nucleus (SCN). The BACHD mice exhibit reduced rhythms in spontaneous electrical activity in SCN neurons. Interestingly, the expression of the clock gene PERIOD2 was not altered in the SCN of the BACHD line. Together, this data is consistent with the hypothesis that the HD mutations interfere with the expression of robust circadian rhythms in behavior and physiology. The data raise the possibility that the electrical activity within the central clock itself may be altered in this disease.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Fig. 1
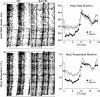
Circadian dysfunction is a common feature of mouse models of HD. Mice were placed individually in cages with running wheels, and locomotor activity was recorded under different lighting conditions. Each horizontal row represents an activity record for a 24-hr day. Successive days are plotted from top to bottom. The grey shading represents darkness. Mice were initially held in LD (12:12) and then released into DD. Panels show examples of the wheel-running activity recorded from WT (A), BACHD (B) , R6/2 CAG 250 (C), R6/2 CAG 140 (D), and CAG 140 (E). The mice were all 2-3 mo of age. See Table 1 for detailed analysis.
Fig. 2
BACHD mice show an age-related decline in activity levels. Panels show examples of wheel-running activity measured from WT (left panels) and littermate BACHD (middle panels) measured at ~3, 6, 9, and 12 mo of age. The average waveform of activity for each genotype (black line =WT; grey line = BACHD) as measured over 10-days in LD is also shown (right panels). Activity and power showed progressive declines under both LD and DD conditions. See Table 2 for detailed analysis.
Fig. 3
BACHD mice show a decreased circadian response to light. (A) Examples of the response to 6 hr shifts in the LD cycle for WT and BACHD mice at ~6 mo of age. (B) Examples of light-induced phase shifts for WT and BACHD mice at ~6 mo of age in DD. Mice in DD were exposed to light (100 lux, 10 min duration) at CT 16 (indicated by symbol) and the resulting phase delay measured.
Fig. 4
BACHD mice exhibit a breakdown in circadian rhythms as measured by telemetry. Examples of heart rate and body temperature measured from WT (left panels) and littermate BACHD (right panels) mice at ~6 mo of age are shown. Each horizontal row represents an activity record for a 24-hr day. Activity double plotted to aid detection of activity patterns. Successive days are plotted from top to bottom. The average waveform of activity for each genotype as measured over 10-days in LD is also shown (right panels).
Fig. 5
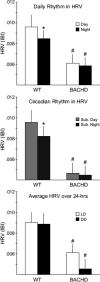
BACHD mice exhibit a loss of their circadian rhythm in HRV. HRV is determined by calculating the variance of the time between individual beats, and is also known as the inter-beat-interval (IBI). Top panel shows the average HRV as measured in the day and in the night when mice are maintained on an LD cycle. The middle panel shows the average HRV as measured in the subjective day and night under DD conditions. The bottom panel shows that the overall HRV was significantly reduced in the BACHD mice. The * indicates significance difference in the HRV at the level of P < 0.05 as analyzed by one-way ANOVA followed by Tukey's post-hoc comparison.
Fig. 6
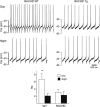
Daytime spontaneous neural activity is reduced in the SCN of BACHD mice. Using the current-clamp recording technique in the cell-attached configuration, we measured the spontaneous firing rate (SFR) in dorsal SCN neurons during the day (ZT 4-6; n = 13-14 per genotype) and night (ZT 16-18; n = 12 per genotype). The top panels show representative examples of firing rate recorded from the WT and BACHD mice at each time point. The bottom panel shows plots of average firing rate for each genotype. Data is shown at means ± SEM. The * indicates significance difference between SFR at the level of P < 0.05 as analyzed by two-way ANOVA followed by Tukey's post-hoc comparison.
Fig. 7
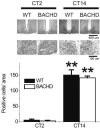
PER2 rhythm within the SCN did not appear to be disrupted even in aged BACHD mice. Mice were held in DD and wheel running activity measured to determine circadian phase. IHC was used to measure PER2 immunoreactivity in the SCN (n = 3-4 per group) of BACHD and WT controls. Tissue was collected in subjective day (CT 2) or subjective night (CT 14). (top panels) Photomicrographs of SCN tissue of each genotype in low (10X) and higher (40X) magnification. (bottom panel) Numbers of PER2 immuno-positive cells in the SCN varied as a function of time of day with highest count in early night. No differences were found between the genotypes. Tukey's post-hoc comparison, **P<0.01 (vs. CT2).
Similar articles
- Degeneration of ipRGCs in Mouse Models of Huntington's Disease Disrupts Non-Image-Forming Behaviors Before Motor Impairment.
Lin MS, Liao PY, Chen HM, Chang CP, Chen SK, Chern Y. Lin MS, et al. J Neurosci. 2019 Feb 20;39(8):1505-1524. doi: 10.1523/JNEUROSCI.0571-18.2018. Epub 2018 Dec 26. J Neurosci. 2019. PMID: 30587542 Free PMC article. - Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease.
Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Morton AJ, et al. J Neurosci. 2005 Jan 5;25(1):157-63. doi: 10.1523/JNEUROSCI.3842-04.2005. J Neurosci. 2005. PMID: 15634777 Free PMC article. - Circadian-based Treatment Strategy Effective in the BACHD Mouse Model of Huntington's Disease.
Whittaker DS, Loh DH, Wang HB, Tahara Y, Kuljis D, Cutler T, Ghiani CA, Shibata S, Block GD, Colwell CS. Whittaker DS, et al. J Biol Rhythms. 2018 Oct;33(5):535-554. doi: 10.1177/0748730418790401. Epub 2018 Aug 7. J Biol Rhythms. 2018. PMID: 30084274 - Do Disruptions in the Circadian Timing System Contribute to Autonomic Dysfunction in Huntington's Disease?
Park S, Colwell CS. Park S, et al. Yale J Biol Med. 2019 Jun 27;92(2):291-303. eCollection 2019 Jun. Yale J Biol Med. 2019. PMID: 31249490 Free PMC article. Review. - Sleep and Circadian Rhythm Dysfunction in Animal Models of Huntington's Disease.
Morton AJ. Morton AJ. J Huntingtons Dis. 2023;12(2):133-148. doi: 10.3233/JHD-230574. J Huntingtons Dis. 2023. PMID: 37334613 Free PMC article. Review.
Cited by
- Sleep and circadian dysfunction in neurodegenerative disorders: insights from a mouse model of Huntington's disease.
Kuljis D, Schroeder AM, Kudo T, Loh DH, Willison DL, Colwell CS. Kuljis D, et al. Minerva Pneumol. 2012 Sep;51(3):93-106. Minerva Pneumol. 2012. PMID: 23687390 Free PMC article. - Degeneration of ipRGCs in Mouse Models of Huntington's Disease Disrupts Non-Image-Forming Behaviors Before Motor Impairment.
Lin MS, Liao PY, Chen HM, Chang CP, Chen SK, Chern Y. Lin MS, et al. J Neurosci. 2019 Feb 20;39(8):1505-1524. doi: 10.1523/JNEUROSCI.0571-18.2018. Epub 2018 Dec 26. J Neurosci. 2019. PMID: 30587542 Free PMC article. - A 24-Hour Study of the Hypothalamo-Pituitary Axes in Huntington's Disease.
Kalliolia E, Silajdžić E, Nambron R, Costelloe SJ, Martin NG, Hill NR, Frost C, Watt HC, Hindmarsh P, Björkqvist M, Warner TT. Kalliolia E, et al. PLoS One. 2015 Oct 2;10(10):e0138848. doi: 10.1371/journal.pone.0138848. eCollection 2015. PLoS One. 2015. PMID: 26431314 Free PMC article. - Membrane Currents, Gene Expression, and Circadian Clocks.
Allen CN, Nitabach MN, Colwell CS. Allen CN, et al. Cold Spring Harb Perspect Biol. 2017 May 1;9(5):a027714. doi: 10.1101/cshperspect.a027714. Cold Spring Harb Perspect Biol. 2017. PMID: 28246182 Free PMC article. Review. - Sex differences in sleep architecture in a mouse model of Huntington's disease.
Chiem E, Zhao K, Stark G, Ghiani CA, Colwell CS, Paul KN. Chiem E, et al. J Neurosci Res. 2024 Jan;102(1):e25290. doi: 10.1002/jnr.25290. J Neurosci Res. 2024. PMID: 38284849 Free PMC article.
References
- Aziz N, Anguelova G, Marinus J, Lammers G, Roos R. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington's disease. Parkinsonism Relat Disord. 2010;16:345–350. - PubMed
- Bigger JJ, Fleiss J, Steinman R, Rolnitzky L, Kleiger R, Rottman J. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. - PubMed
- Bray M, Shaw C, Moore M, Garcia R, Zanquetta M, Durgan D, Jeong W, Tsai J, Bugger H, Zhang D, Rohrwasser A, Rennison J, Dyck J, Litwin S, Hardin P, Chow C, Chandler M, Abel E, Young M. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–1047. - PubMed
- Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, Basile F, Silveri N. Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur Rev Med Pharmacol Sci. 2009;13:299–307. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases