Human adrenal cells that express both 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production - PubMed (original) (raw)
Human adrenal cells that express both 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production
Yasuhiro Nakamura et al. J Steroid Biochem Mol Biol. 2011 Feb.
Abstract
Androstenedione is one of several weak androgens produced in the human adrenal gland. 3β-Hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) are both required for androstenedione production. However, previous studies demonstrated the expression of HSD3B2 within the zona glomerulosa (ZG) and fasciculata (ZF) but low levels in the zona reticularis. In contrast, CYB5A expression increases in the zona reticularis (ZR) in human adrenal glands. Although their colocalization has been reported in gonadal theca and Leydig cells this has not been studied in the human adrenal. Therefore, we immonolocalized HSD3B2 and CYB5A in normal human adrenal glands and first demonstrated their co-expression in the cortical cells located at the border between the ZF and ZR in normal human adrenal. Results of in vitro studies using the human adrenal H295R cells treated with the HSD3B2 inhibitor, trilostane, also demonstrated a markedly decreased androstenedione production. Decreasing CYB5A mRNA using its corresponding siRNA also resulted in significant inhibition of androstenedione production in the H295R cells. These findings together indicate that there are a group of cells co-expressing HSD3B2 and CYB5A with hybrid features of both ZF and ZR in human adrenal cortex, and these hybrid cortical cells may play an important role in androstenedione production in human adrenal gland.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Potential pathways of androstenedione synthesis in the human adrenal gland. Cholesterol is converted to pregnenolone by CYP11A1. Conversion of pregnenolone to androstenedione might take place by either of two pathways. In the Δ5 pathway, pregnenolone undergoes 17α-hydroxylation to 17α-hydroxypregnenolone and scission of the C17-C20 bond to yield DHEA, both catalyzed by CYP17. HSD3B2 can also convert each Δ5 steroid to its corresponding Δ4 steroid, including androstenedione. For progesterone to be converted to testosterone, it must follow a Δ4 pathway through 17α-hydroxyprogesterone and to androstenedione. However, CYP17 in human adrenal gland have high 17, 20-lyase activity only in the Δ5 pathway ($). This figure was reproduced based on a previous study [5].
Figure 2
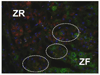
The localization of CYB5A and HSD3B2 in the human adult adrenal gland. Immunopositive cells for CYB5A appear red, and immunopositive cells for HSD3B2 appear green. Double-immunopositive cells are detected in the border between the ZF and ZR (circles). The nuclei are stained with 4',6-diamidino-2-phenylindole (DAPI) (blue). We performed these double immunofluorescence analysis at least three sets of the human adrenal glands.(ZF: zona fasciculata; ZR: zona reticularis).
Figure 3
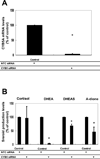
Effects of siRNA depletion of CYB5A on adrenal cell production of adrenal steroids. Panel A. H295R adrenal cells were transfected with or without siRNA against CYB5A (CYB5 siRNA) or Stealth™ RNAi Negative Control Duplexes (Control). After 48 h, mRNA for CYB5A was detected by qPCR. 18s rRNA expression were used for normalization. Data are presented as mean ± standard error (*: P < 0.05). Panel B. The level of cortisol, DHEA, DHEAS, and androstenedione in the media with H295R cells at 48 h after double transfection of either CYB5A (CYB5 siRNA) or Stealth™ RNAi Negative Control Duplexes (NTC). Data are presented as mean ± standard error (*: P < 0.05). We performed three independent experiments.
Figure 4
Effects of trilostane on adrenal cell production of steroids. The amount of cortisol, DHEA, DHEAS, and androstenedione in the media with H295R cells was determined after 48h incubation with or without trilostane (10 µM). Data are presented as mean ± standard error (*: P < 0.05). We performed three independent experiments.
Similar articles
- 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging.
Nakamura Y, Fujishima F, Hui XG, Felizola SJ, Shibahara Y, Akahira J, McNamara KM, Rainey WE, Sasano H. Nakamura Y, et al. Endocr Res. 2015;40(1):8-13. doi: 10.3109/07435800.2014.895377. Epub 2014 May 15. Endocr Res. 2015. PMID: 24832628 Free PMC article. - The Age-Dependent Changes of the Human Adrenal Cortical Zones Are Not Congruent.
Tezuka Y, Atsumi N, Blinder AR, Rege J, Giordano TJ, Rainey WE, Turcu AF. Tezuka Y, et al. J Clin Endocrinol Metab. 2021 Apr 23;106(5):1389-1397. doi: 10.1210/clinem/dgab007. J Clin Endocrinol Metab. 2021. PMID: 33524149 Free PMC article. - Age-dependent Increases in Adrenal Cytochrome b5 and Serum 5-Androstenediol-3-sulfate.
Rege J, Karashima S, Lerario AM, Smith JM, Auchus RJ, Kasa-Vubu JZ, Sasano H, Nakamura Y, White PC, Rainey WE. Rege J, et al. J Clin Endocrinol Metab. 2016 Dec;101(12):4585-4593. doi: 10.1210/jc.2016-2864. Epub 2016 Sep 13. J Clin Endocrinol Metab. 2016. PMID: 27623070 Free PMC article. - Adrenal changes associated with adrenarche.
Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Nakamura Y, et al. Rev Endocr Metab Disord. 2009 Mar;10(1):19-26. doi: 10.1007/s11154-008-9092-2. Rev Endocr Metab Disord. 2009. PMID: 18821019 Free PMC article. Review. - Regulation of the adrenal androgen biosynthesis.
Rainey WE, Nakamura Y. Rainey WE, et al. J Steroid Biochem Mol Biol. 2008 Feb;108(3-5):281-6. doi: 10.1016/j.jsbmb.2007.09.015. Epub 2007 Sep 11. J Steroid Biochem Mol Biol. 2008. PMID: 17945481 Free PMC article. Review.
Cited by
- Identification of candidate reference chemicals for in vitro steroidogenesis assays.
Pinto CL, Markey K, Dix D, Browne P. Pinto CL, et al. Toxicol In Vitro. 2018 Mar;47:103-119. doi: 10.1016/j.tiv.2017.11.003. Epub 2017 Nov 13. Toxicol In Vitro. 2018. PMID: 29146384 Free PMC article. Review. - Retinoic acid receptor beta and angiopoietin-like protein 1 are involved in the regulation of human androgen biosynthesis.
Udhane SS, Pandey AV, Hofer G, Mullis PE, Flück CE. Udhane SS, et al. Sci Rep. 2015 May 13;5:10132. doi: 10.1038/srep10132. Sci Rep. 2015. PMID: 25970467 Free PMC article. - Morphologic and Molecular Characterization of Adrenals and Adrenal Rest Affected by Congenital Adrenal Hyperplasia.
Kolli V, da Cunha IW, Kim S, Iben JR, Mallappa A, Li T, Gaynor A, Coon SL, Quezado MM, Merke DP. Kolli V, et al. Front Endocrinol (Lausanne). 2021 Sep 20;12:730947. doi: 10.3389/fendo.2021.730947. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34616364 Free PMC article. - 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging.
Nakamura Y, Fujishima F, Hui XG, Felizola SJ, Shibahara Y, Akahira J, McNamara KM, Rainey WE, Sasano H. Nakamura Y, et al. Endocr Res. 2015;40(1):8-13. doi: 10.3109/07435800.2014.895377. Epub 2014 May 15. Endocr Res. 2015. PMID: 24832628 Free PMC article. - The role of adrenal derived androgens in castration resistant prostate cancer.
Barnard M, Mostaghel EA, Auchus RJ, Storbeck KH. Barnard M, et al. J Steroid Biochem Mol Biol. 2020 Mar;197:105506. doi: 10.1016/j.jsbmb.2019.105506. Epub 2019 Oct 28. J Steroid Biochem Mol Biol. 2020. PMID: 31672619 Free PMC article. Review.
References
- Odell WD, Parker LN. Control of adrenal androgen production. Endocr. Res. 1984–1985;10:617–630. - PubMed
- Labrie C, Simard J, Zhao HF, Bélanger A, Pelletier G G, Labrie F. Stimulation of androgen-dependent gene expression by the adrenal precursors dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology. 1989;124:2745–2754. - PubMed
- de Ronde W, Hofman A, Pols HA, de Jong FH. A direct approach to the estimation of the origin of oestrogens and androgens in elderly men by comparison with hormone levels in postmenopausal women. Eur. J. Endocrinol. 2005;152:261–268. - PubMed
- Bidlingmaier F, Dörr HG, Eisenmenger W, Kuhnle U, Knorr D. Contribution of the adrenal gland to the production of androstenedione and testosterone during the first two years of life. J. Clin. Endocrinol. Metab. 1986;62:331–335. - PubMed
- Flück CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J. Clin. Endocrinol. Metab. 2003;88:3762–3766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous