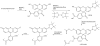Nox2 redox signaling maintains essential cell populations in the brain - PubMed (original) (raw)
Nox2 redox signaling maintains essential cell populations in the brain
Bryan C Dickinson et al. Nat Chem Biol. 2011 Feb.
Abstract
Reactive oxygen species (ROS) are conventionally classified as toxic consequences of aerobic life, and the brain is particularly susceptible to ROS-induced oxidative stress and damage owing to its high energy and oxygen demands. NADPH oxidases (Nox) are a widespread source of brain ROS implicated in seizures, stroke and neurodegeneration. A physiological role for ROS generation in normal brain function has not been established, despite the fact that mice and humans lacking functional Nox proteins have cognitive deficits. Using molecular imaging with Peroxyfluor-6 (PF6), a new selective fluorescent indicator for hydrogen peroxide (H(2)O(2)), we show that adult hippocampal stem/progenitor cells (AHPs) generate H(2)O(2) through Nox2 to regulate intracellular growth signaling pathways, which in turn maintains their normal proliferation in vitro and in vivo. Our results challenge the traditional view that brain ROS are solely deleterious by demonstrating that controlled ROS chemistry is needed for maintaining specific cell populations.
Figures
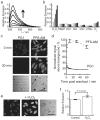
Figure 1. Spectroscopic characterization and cell culture validation of PF6-AM
(a) Fluorescence turn-on response of 5 μM PF6 at 0, 5, 15, 30, 45, and 60 minutes after the addition of 100 μM H2O2. (b) Fluorescence responses of 5 μM PF6 to various reactive oxygen species (ROS). Bars represent relative responses at 0, 5, 15, 30, 45, and 60 min after addition of each ROS. Data shown are for 10 mM O2- (with 10 μM Catalase), 200 μM NO, and 100 μM for all other ROS. (c) HeLa cells were loaded with either 5 μM PG1 or 5 μM PF6-AM for 15 minutes, then washed twice with DPBS and imaged at 0, 10, 30 and 60 minutes post dye washing. (d) Quantification of the experiment as conducted in (c). (e) HeLa cells were loaded with 5 μM PF6-AM for 15 minutes, stimulated with either water carrier or 10 μM H2O2 for 30 minutes, and imaged. (f) Quantification of the experiment as conducted in (e). Statistical analyses were performed with a two-tailed Student's _t_-test and error bars are ± s.e.m. 50 μm scale bars are shown.
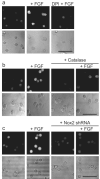
Figure 2. Application of PF6 to demonstrate that adult hippocampal stem/progenitor cells (AHPs) produce H2O2 upon FGF-2 stimulation
(a) After FGF-2 starvation, AHPs were loaded with 5 μM PF6-AM for 30 minutes, stimulated with 20 ng/mL FGF-2 or media for 30 minutes, and then imaged. For DPI treatment, cells were preincubated in media containing 5 μM DPI before FGF-2 stimulation. (b) AHPs were transfected with either Catalase or control vector and treated as in (a). (c) AHPs were transfected with either Nox2-shRNA or control vector and treated as in (a). Brightfield images are shown for each representative image with a 50 μm scale bar.
Figure 3. Cellular redox status affects AHP growth signaling
(a) After FGF-2 starvation, AHPs were stimulated with vehicle control (buffer), 20 ng/mL FGF-2, 300, 500, or 1000 μM H2O2 for 30 min. (b) AHPs transfected with either Catalase or a control vector. After FGF-2 starvation, AHPs were stimulated with 20 ng/mL and lysed at the given time points. (c) After FGF-2 starvation, AHPs were incubated with NAC, DPI, or vehicle control (DMSO) for 40 minutes, then stimulated with 20 ng/mL FGF-2 and lysed at the given time points. (d) Nox2 mRNA detection in AHPs measured by RT-PCR. (e) Nox2 expression of AHP whole cell extracts transfected with either Nox2 shRNA or an empty vector as measured by western blot analysis using either a mouse monoclonal (m) or a rabbit polyclonal (r) Nox2 antibody, followed by stripping and reprobing for actin as a loading control. An arrow shows the band in the Nox2 monoclonal antibody blot that matches the band in the Nox2 polyclonal blot, which corresponds to the molecular weight of Nox2. (f) AHPs transfected with either Nox2 shRNA or the empty vector. (g) AHPs transfected with either Nox2 shRNA or Nox3 shRNA. After 12 hour FGF-2 starvation, AHPs were stimulated with 20 ng/mL and lysed at the given time points. phospho-Akt, phospho-ERK, or mouse monoclonal Nox2 were measured by western blot analysis of whole cell extracts, and blots were stripped and reprobed for total protein or actin as loading controls.
Figure 4. Nox2 is essential for normal proliferation of AHPs in vitro and in vivo
(a) 5-day growth assay of AHPs grown in the presence of FGF and varying concentrations of DPI (n = 4). (b) 5-day growth assay of AHPs transfected with either Nox2 shRNA or the empty vector and grown in the presence of FGF (n = 3). (c) 5-day growth assay of AHPs transfected with either Nox2 shRNA or Nox3 shRNA and grown in the presence of FGF (n = 3). (d) Dentate gyrus of a CL57BL/6J mouse after 7 days of BrdU injections. (e) Dentate gyrus of a Nox2-/- mouse after 7 days of BrdU injections with 100 μm scale bar. (f) Example of a cluster of BrdU/Sox2 positive AHPs in a CL57BL/6J control after seven days of BrdU injections. (g) Example of a single BrdU/Sox2 positive cell in a Nox2-/- mouse after 7 days of BrdU injections with 20 μm scale bar. Sections stained for BrdU (blue), NeuN (red) and Sox2 (green). (h) Quantification of BrdU/Sox2 positive cells in either control or Nox2-/- mice after 7 days of BrdU injections (n = 5). (i) Example of newborn neurons in a CL57BL/6J control mouse 28 days after 7 days of BrdU injections. (j) Example of a newborn neuron in a Nox2-/- mouse 28 days after 7 days of BrdU injections with 20 μm scale bar. (k) Quantification of BrdU/NeuN positive cells in either control or Nox2-/- mice 28 days after 7 days of BrdU injections (n = 4). For all panels data were normalized to controls and statistical analyses were performed with a two-tailed Student's _t_-test. *P ≤ 0.05, **P ≤ 0.005 and error bars are ± s.e.m.
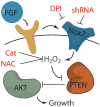
Figure 5. Model for the role of Nox2 in FGF-2 redox signaling in AHPs
The mitogen FGF-2 induces the production of H2O2 in AHPs, which can be blocked by either the general flavin inhibitor DPI, the antioxidant NAC, the expression of Catalase or genetic manipulation of Nox2. Nox2-generated H2O2 oxidizes and deactivates PTEN, which enhances signaling through Akt and manifest phenotypes in growth rates of AHPs in vitro and in vivo.
Scheme 1. Design and synthesis of Peroxyfluor-6 Acetoxymethyl Ester, PF6-AM
Similar articles
- Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems.
Lippert AR, Van de Bittner GC, Chang CJ. Lippert AR, et al. Acc Chem Res. 2011 Sep 20;44(9):793-804. doi: 10.1021/ar200126t. Epub 2011 Aug 11. Acc Chem Res. 2011. PMID: 21834525 Free PMC article. - ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis.
Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, Ushio-Fukai M. Kim YM, et al. Am J Physiol Cell Physiol. 2017 Jun 1;312(6):C749-C764. doi: 10.1152/ajpcell.00346.2016. Epub 2017 Apr 19. Am J Physiol Cell Physiol. 2017. PMID: 28424170 Free PMC article. - Oxidants in Physiological Processes.
Knaus UG. Knaus UG. Handb Exp Pharmacol. 2021;264:27-47. doi: 10.1007/164_2020_380. Handb Exp Pharmacol. 2021. PMID: 32767144 Review. - Redox regulation of Nox proteins.
Pendyala S, Natarajan V. Pendyala S, et al. Respir Physiol Neurobiol. 2010 Dec 31;174(3):265-71. doi: 10.1016/j.resp.2010.09.016. Epub 2010 Sep 29. Respir Physiol Neurobiol. 2010. PMID: 20883826 Free PMC article. Review. - NOX Inhibitors: From Bench to Naxibs to Bedside.
Elbatreek MH, Mucke H, Schmidt HHHW. Elbatreek MH, et al. Handb Exp Pharmacol. 2021;264:145-168. doi: 10.1007/164_2020_387. Handb Exp Pharmacol. 2021. PMID: 32780287
Cited by
- Imaging beyond the proteome.
Chang PV, Bertozzi CR. Chang PV, et al. Chem Commun (Camb). 2012 Sep 14;48(71):8864-79. doi: 10.1039/c2cc31845h. Epub 2012 Jul 17. Chem Commun (Camb). 2012. PMID: 22801420 Free PMC article. Review. - Mitochondrial alarmins released by degenerating motor axon terminals activate perisynaptic Schwann cells.
Duregotti E, Negro S, Scorzeto M, Zornetta I, Dickinson BC, Chang CJ, Montecucco C, Rigoni M. Duregotti E, et al. Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):E497-505. doi: 10.1073/pnas.1417108112. Epub 2015 Jan 20. Proc Natl Acad Sci U S A. 2015. PMID: 25605902 Free PMC article. - Reaction-based small-molecule fluorescent probes for chemoselective bioimaging.
Chan J, Dodani SC, Chang CJ. Chan J, et al. Nat Chem. 2012 Dec;4(12):973-84. doi: 10.1038/nchem.1500. Nat Chem. 2012. PMID: 23174976 Free PMC article. Review. - Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts.
Milkovic L, Cipak Gasparovic A, Cindric M, Mouthuy PA, Zarkovic N. Milkovic L, et al. Cells. 2019 Jul 30;8(8):793. doi: 10.3390/cells8080793. Cells. 2019. PMID: 31366062 Free PMC article. Review. - Embryonic anti-aging niche.
Conboy IM, Yousef H, Conboy MJ. Conboy IM, et al. Aging (Albany NY). 2011 May;3(5):555-63. doi: 10.18632/aging.100333. Aging (Albany NY). 2011. PMID: 21666284 Free PMC article.
References
- Floyd RA. Oxidative damage to behavior during aging. Science. 1991;254:1597–1597. - PubMed
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nature Med. 2004;10:S18–25. - PubMed
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. - PubMed
Publication types
MeSH terms
Grants and funding
- GM 79465/GM/NIGMS NIH HHS/United States
- R21 EB007295/EB/NIBIB NIH HHS/United States
- T32 GM066698/GM/NIGMS NIH HHS/United States
- EB 007295/EB/NIBIB NIH HHS/United States
- R01 GM079465-05/GM/NIGMS NIH HHS/United States
- R01 GM079465/GM/NIGMS NIH HHS/United States
- R01 GM079465-04/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous