Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema - PubMed (original) (raw)
. 2011 Jan 11;108(2):846-51.
doi: 10.1073/pnas.1015217108. Epub 2010 Dec 27.
Phillip M Rappold, Takumi Fujita, Arnulfo Torres, Lane K Bekar, Takahiro Takano, Weiguo Peng, Fushun Wang, Vinita Rangroo Thrane, Rune Enger, Nadia N Haj-Yasein, Øivind Skare, Torgeir Holen, Arne Klungland, Ole P Ottersen, Maiken Nedergaard, Erlend A Nagelhus
Affiliations
- PMID: 21187412
- PMCID: PMC3021020
- DOI: 10.1073/pnas.1015217108
Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema
Alexander S Thrane et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Aquaporin-4 (AQP4) is a primary influx route for water during brain edema formation. Here, we provide evidence that brain swelling triggers Ca(2+) signaling in astrocytes and that deletion of the Aqp4 gene markedly interferes with these events. Using in vivo two-photon imaging, we show that hypoosmotic stress (20% reduction in osmolarity) initiates astrocytic Ca(2+) spikes and that deletion of Aqp4 reduces these signals. The Ca(2+) signals are partly dependent on activation of P2 purinergic receptors, which was judged from the effects of appropriate antagonists applied to cortical slices. Supporting the involvement of purinergic signaling, osmotic stress was found to induce ATP release from cultured astrocytes in an AQP4-dependent manner. Our results suggest that AQP4 not only serves as an influx route for water but also is critical for initiating downstream signaling events that may affect and potentially exacerbate the pathological outcome in clinical conditions associated with brain edema.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
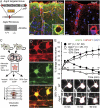
Aqp4 KO strategy and validation. (A) The targeted allele contained a flippase recognition target (FRT)-neomycin-FRT cassette after exon 3 and LoxP sites upstream and downstream of exons 1–3. Floxed mice were bred with Cre-expressing mice to produce mice with the Aqp4 KO allele. Western blot confirmed the absence of AQP4 in _Aqp4_−/− mice. (B) Immunofluorescence micrographs of mouse cortex probed with primary antibodies against AQP4 (green) and GFAP (red) with DAPI-labeled nuclei (blue) for orientation. The AQP4 immunofluorescence signal is absent in _Aqp4_−/− mice. Insets display perivascular AQP4 and GFAP labeling at higher magnification. (Scale bar: 25 μm; Inset, 5 μm.) (C) Experimental design for validating the effect of Aqp4 deletion on osmotically induced astrocyte swelling. Acute brain slices were prepared from WT and _Aqp4_−/− mouse pups. Slices were loaded with Texas red hydrazide and perfused with aCSF with normal (isotonic) or reduced osmolarity (−20% or −30% Osm). Sectional images were acquired for 3D volume analysis. (D) Two-photon imaging of Texas red hydrazide-loaded slices obtained from mice expressing GFP in astrocytes (_Glt-1_–EGFP BAC transgenic mice) confirmed that the dye was selectively taken up by astrocytes. (Scale bar: 5 μm.) (E) Exposure of acute cortical slices to 20% reduction in osmolarity (−20% Osm) induced more prominent swelling of astrocytic somata in WT (n = 37) than in _Aqp4_−/− mice (n = 26; P < 0.001 at 5 min, two-tailed Student t test). The initial swelling was followed by shrinkage reflecting regulatory volume decrease. More severe osmotic stress (−30% Osm) induced continuous swelling in both genotypes (n = 30 and 31). Error bars represent SEM. Lower shows representative images of astrocytes exposed to −20% Osm. The red ring marks the astrocyte soma circumference at baseline. (Scale bar: 5 μm.)
Fig. 2.
In vivo two-photon imaging of astroglial Ca2+ signals during hypoosmotic stress. (A) Diagram of experimental setup. Astrocytic Ca2+ transients were detected in anesthetized _Glt-1_–EGFP BAC transgenic mice after loading of Rhod2 AM onto the cortical surface. (B) Images show Rhod2 fluorescence in a GFP-expressing astrocyte. Because increase in Rhod2 signals were not associated with changes in GFP fluorescence, the ratio between the two signals provided a measure of the astrocytic Ca2+ signal. (C) During brain swelling, the ratio between Rhod2 and GFP fluorescent signal intensities (lower trace) was less sensitive to inadvertent small shifts in focal plane than Rhod2 signals (upper trace). Thus, Rhod2/GFP signals were used for reliably defining Ca2+ spikes [i.e., transients exceeding 20% (dashed line) of baseline]. (D) Pooled Ca2+ traces (measured as relative changes in Rhod2/GFP) for all astrocytes (n = 24) within an image field in a WT mouse subjected to i.p. water injection (indicated by arrow; 200 mL/kg) to induce osmotic brain swelling. Note increase in spike frequency and amplitude as brain edema develops. (E) In WT mice, relative spike amplitude and proportion of spikes lasting ≥30 s were higher in the last 15 min than in the first 15 min after water injection. (F) Traces of relative Ca2+ changes in WT astrocytes 30 min after i.p. water injection. The position of the respective astrocytes (confluent Rhod2 and GFP signals in yellow) is indicated in Left. FITC-dextran (green) was injected i.v. at the beginning of the experiment to outline the vasculature, and it confirmed vascular perfusion. Time-lapse sequence of the Ca2+ responses is shown in Right. Note the intense and long-lasting Ca2+ surge in the astrocytic soma and endfoot surrounding a vein. (Scale bar: 25 μm.) (G) In WT mice, the frequency of astrocytic Ca2+ spikes was higher already in the first 15 min after water injection compared with the control state, and it was even higher in the last 15 min of observation. In _Aqp4_−/− mice, water injection did not increase spike frequency. The percentage of astrocytes with more than or equal to one Ca2+ spike(s) per 15-min observation (active astrocytes) increased profoundly in WT mice after water injection. In _Aqp4_−/− mice, the number of active astrocytes increased only at the late phase of osmotic brain swelling. Error bars represent SEM. Mixed model analyses were performed using a binomial (Bernoulli) model with logit link for binary observations (passive or active cells), a Poisson model for count data (spike frequency), and a linear model for spike amplitudes (24).
Fig. 3.
Osmotically induced astrocytic Ca2+ responses and ATP release in vitro. (A) Two-photon images of Rhod2 AM-loaded acute cortical slices obtained from WT (Upper) and _Aqp4_−/− (Lower) mice. Traces shown are from astrocytes marked in Left. Exposure of slices from WT mice to aCSF with 20% reduction in osmolarity (−20% Osm) induced brisk astrocytic Ca2+ spikes. In contrast, this osmotic stress failed to elicit Ca2+ spikes in most AQP4-deficient astrocytes. (B) Quantitative analysis of astrocytic Ca2+ responses to osmotic stress. Deletion of Aqp4 or blocking P2 purinergic receptors with PPADS/suramin significantly reduced the number of astrocytes that responded with Ca2+ spikes during exposure to −20% Osm. When more severe hypoosmotic stress (−30% Osm) was applied, a larger fraction of the _Aqp4_−/− astrocytes responded. The amplitude of the Ca2+ spikes differed between the genotypes for both types of stress. Image shows intense Ca2+ signals in Rhod2-loaded astrocytes after microinjection of ATP and FITC-dextran (green; to verify injection) into the slice. Representative traces from WT and _Aqp4_−/− mice are shown. (C) Cultured astrocytes exposed to hypoosmotic media (−20% Osm, 15 min) released significantly more ATP than those kept in isotonic media. Astrocytes from _Aqp4_−/− mice did not show osmotically induced ATP release. P values were obtained by two-tailed Student t test. Error bars represent SEM. (Scale bar: 25 μm.) (D) Diagram showing proposed involvement of AQP4 in astrocyte signaling cascades during hypoosmotic stress. AQP4-mediated water influx triggers Ca2+ transients, partly by promoting release of ATP and activation of P2 purinergic receptors.
Comment in
- Glia: aquaporin: not so swell?
Welberg L. Welberg L. Nat Rev Neurosci. 2011 Feb;12(2):66. doi: 10.1038/nrn2984. Nat Rev Neurosci. 2011. PMID: 21309098 No abstract available.
Similar articles
- Astroglial endfeet exhibit distinct Ca2+ signals during hypoosmotic conditions.
Eilert-Olsen M, Hjukse JB, Thoren AE, Tang W, Enger R, Jensen V, Pettersen KH, Nagelhus EA. Eilert-Olsen M, et al. Glia. 2019 Dec;67(12):2399-2409. doi: 10.1002/glia.23692. Epub 2019 Jul 27. Glia. 2019. PMID: 31350866 - Activated microglia-derived macrophage-like cells exacerbate brain edema after ischemic stroke correlate with astrocytic expression of aquaporin-4 and interleukin-1 alpha release.
Murata Y, Sugimoto K, Yang C, Harada K, Gono R, Harada T, Miyashita Y, Higashisaka K, Katada R, Tanaka J, Matsumoto H. Murata Y, et al. Neurochem Int. 2020 Nov;140:104848. doi: 10.1016/j.neuint.2020.104848. Epub 2020 Sep 11. Neurochem Int. 2020. PMID: 32920036 - Three distinct roles of aquaporin-4 in brain function revealed by knockout mice.
Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Verkman AS, et al. Biochim Biophys Acta. 2006 Aug;1758(8):1085-93. doi: 10.1016/j.bbamem.2006.02.018. Epub 2006 Mar 10. Biochim Biophys Acta. 2006. PMID: 16564496 Review. - Aquaporin-4 and brain edema.
Papadopoulos MC, Verkman AS. Papadopoulos MC, et al. Pediatr Nephrol. 2007 Jun;22(6):778-84. doi: 10.1007/s00467-006-0411-0. Epub 2007 Mar 9. Pediatr Nephrol. 2007. PMID: 17347837 Free PMC article. Review.
Cited by
- Functional characterization of a novel aquaporin from Dictyostelium discoideum amoebae implies a unique gating mechanism.
von Bülow J, Müller-Lucks A, Kai L, Bernhard F, Beitz E. von Bülow J, et al. J Biol Chem. 2012 Mar 2;287(10):7487-94. doi: 10.1074/jbc.M111.329102. Epub 2012 Jan 18. J Biol Chem. 2012. PMID: 22262860 Free PMC article. - Protective role of brain water channel AQP4 in murine cerebral malaria.
Promeneur D, Lunde LK, Amiry-Moghaddam M, Agre P. Promeneur D, et al. Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):1035-40. doi: 10.1073/pnas.1220566110. Epub 2012 Dec 31. Proc Natl Acad Sci U S A. 2013. PMID: 23277579 Free PMC article. - Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling.
Lafrenaye AD, Simard JM. Lafrenaye AD, et al. Int J Mol Sci. 2019 Jan 15;20(2):330. doi: 10.3390/ijms20020330. Int J Mol Sci. 2019. PMID: 30650535 Free PMC article. Review. - Astroglial toxicity promotes synaptic degeneration in the thalamocortical circuit in frontotemporal dementia with GRN mutations.
Marsan E, Velmeshev D, Ramsey A, Patel RK, Zhang J, Koontz M, Andrews MG, de Majo M, Mora C, Blumenfeld J, Li AN, Spina S, Grinberg LT, Seeley WW, Miller BL, Ullian EM, Krummel MF, Kriegstein AR, Huang EJ. Marsan E, et al. J Clin Invest. 2023 Mar 15;133(6):e164919. doi: 10.1172/JCI164919. J Clin Invest. 2023. PMID: 36602862 Free PMC article. - Drowning stars: reassessing the role of astrocytes in brain edema.
Thrane AS, Rangroo Thrane V, Nedergaard M. Thrane AS, et al. Trends Neurosci. 2014 Nov;37(11):620-8. doi: 10.1016/j.tins.2014.08.010. Epub 2014 Sep 15. Trends Neurosci. 2014. PMID: 25236348 Free PMC article. Review.
References
- Nase G, Helm PJ, Enger R, Ottersen OP. Water entry into astrocytes during brain edema formation. Glia. 2008;56:895–902. - PubMed
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. - PubMed
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS056188/NS/NINDS NIH HHS/United States
- R01 NS078304/NS/NINDS NIH HHS/United States
- TL1 RR024135/RR/NCRR NIH HHS/United States
- P01 NS050315/NS/NINDS NIH HHS/United States
- R01 NS075177/NS/NINDS NIH HHS/United States
- R01 NS056188/NS/NINDS NIH HHS/United States
- R01 NS078167/NS/NINDS NIH HHS/United States
- P01NS050315/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous