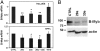miR-29 and miR-30 regulate B-Myb expression during cellular senescence - PubMed (original) (raw)
miR-29 and miR-30 regulate B-Myb expression during cellular senescence
Ivan Martinez et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Cellular senescence is a form of irreversible growth arrest and a major tumor suppressor mechanism. We show here that the miR-29 and miR-30 microRNA families are up-regulated during induced and replicative senescence and that up-regulation requires activation of the Rb pathway. Expression of a reporter construct containing the 3'UTR of the B-Myb oncogene is repressed during senescence, and repression is blocked by mutations in conserved miR-29 and miR-30 binding sites in the B-Myb 3'UTR. In proliferating cells, transfection of miR-29 and miR-30 represses a reporter construct containing the wild-type but not the mutant B-Myb 3'UTR, and repression of the mutant 3'UTR is reinstituted by compensatory mutations in miR-29 and miR-30 that restore binding to the mutant sites. miR-29 and miR-30 introduction also represses expression of endogenous B-Myb and inhibits cellular DNA synthesis. Finally, interference with miR-29 and miR-30 expression inhibits senescence. These findings demonstrate that miR-29 and miR-30 regulate B-Myb expression by binding to its 3'UTR and suggest that these microRNAs play an important role in Rb-driven cellular senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
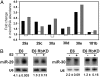
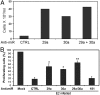
Fig. 1.
Rb-dependent up-regulation of miR-29 and miR-30 microRNAs during senescence in HeLa cell lines. (A) Differential expression of miR-29 and miR-30 family members as measured by TaqMan® microRNA microarray. Fold change compared to uninfected cells was measured for the indicated microRNAs in HeLa/E6 (black bars), HeLa/E6-E7 (white bars), and HeLa/E6-E7Δ (gray bars) cells 4 d after infection with Pava1. miR-181b is an example of a microRNA whose expression did not change during senescence. Similar results were obtained in two independent experiments, the average of which is shown here. (B) Confirmation of Rb-dependent up-regulation of miR-29 and miR-30 by Northern blot analysis. E6 indicates HeLa/E6 cells, and E6 RbKD indicates HeLa/E6 cells stably expressing shRNAs that knockdown expression of p105Rb, p107, and p130 (8). The portion of the gel corresponding to mature microRNAs is shown. In each panel, the left lane contains RNA from uninfected cells, and the right lane contains RNA from cells 6 d after infection with Pava1. The numbers underneath the panels show the average fold induction of microRNA expression by the E2 protein in two independent experiments, +/- standard deviations, normalized to U6 RNA expression.
Fig. 2.
miR-29 and miR-30 binding sites in the 3′UTR of the B-Myb gene. (Top) Map of human B-Myb mRNA, together with the nucleotide number of various landmarks relative to the transcription start site. The B-Myb 3′UTR contains conserved sites complementary to the seed sequences of miR-30, miR-29, and miR-143. The line above the 3′UTR shows the segment cloned into the luciferase reporter plasmid. The sequences of miR-29a and miR-30e and their putative binding sites in the B-Myb 3′UTR are shown below the map. (Left to Right) The top strand in each pair is shown in the 5′ to 3′ orientation, and the bottom strand is shown the 3′ to 5′ orientation, with predicted base pairing between the microRNA seed region and the 3′UTR indicated by vertical lines. The wild-type seed sequence and complementary bases in the 3′UTR are shown in blue; mutations in the binding sites that disrupt base pairing and mutations in the microRNAs that restore it are shown in red.
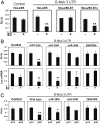
Fig. 3.
Role of the miR-29 and miR-30 binding sites in regulation by the B-Myb 3′UTR. (A) HeLa/E6, HeLa/E6-E7, and HeLa/E6-E7Δ cells were cotransfected with a firefly luciferase plasmid containing a segment of the B-Myb ORF (control) or the wild-type B-Myb 3′UTR, together with a Renilla luciferase plasmid for normalization of transfection efficiency. The day after transfection, cells were mock-infected (-) or infected with Pava1 (+) to induce senescence. Firefly luciferase activity was measured and normalized to Renilla luciferase after an additional 5 d. RLUs, relative light units. For panels A and B, two-tailed _t_-test results are indicated by * for P < 0.05 and ** for P < 0.01, relative to mock-infected cells. Similar results were obtained in three independent experiments. (B) HeLa/E6 cells were transfected with a luciferase plasmid containing the B-Myb ORF fragment (control) or the B-Myb 3′UTR with no mutations (wild type), mutations in the miR-29 binding site alone (miR-29M), mutations in the miR-30 binding site alone (miR-30M), or mutations in both binding sites (29M/30M), as indicated. The day after transfection, cells were either mock-infected (-) or infected with Pava1 (+) to induce senescence. Five days later, firefly luciferase activity was measured and normalized to expression of Renilla luciferase from a cotransfected plasmid (Top), or the amount of firefly luciferase mRNA was measured by qRT-PCR and reported as fold-change relative to luciferase mRNA in uninfected cells transfected with the control plasmid (Bottom). Similar results were obtained in three (Top) or two (Bottom) independent experiments. (C) Passage 13 and passage 35 HFFs were transfected with the control luciferase plasmid or the plasmid containing the B-Myb 3′UTR with no mutations or with mutations in the miR-29 and miR-30 binding sites as described in the legend to B. After 2 d, firefly luciferase activity was measured and normalized to expression of Renilla luciferase from a cotransfected plasmid. Two-tailed _t_-test results are indicated by * for P < 0.05 and ** for P < 0.01, relative to low passage cells. Similar results were obtained in two independent experiments.
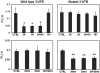
Fig. 4.
Genetic analysis of the interaction between the B-Myb 3′UTR and miR-29 and miR-30. Proliferating HeLa/E6 cells were transfected with 80 nM (total) scrambled control microRNA (CTRL), miR-181b (181), or wild-type (Top) or mutant (Bottom) miR-29a and/or miR-30e that contain compensatory mutations in the seed sequence that restore binding to the mutant binding sites in the B-Myb 3′UTR (see Fig. 2). The next day, cells were transfected with the reporter plasmid containing the B-Myb 3′UTR with no mutations (Left) or with mutations in both microRNA binding sites (Right). Normalized firefly luciferase activity was measured 2 d later. Two-tailed t test results are indicated by * for P < 0.05 and ** for P < 0.01, relative to cells transfected with CTRL microRNA. Similar results were obtained in three (Top) or two (Bottom) independent experiments.
Fig. 5.
Effect of exogenous miR-29a and miR-30e on the expression of B-Myb. (A) Proliferating HeLa/E6 cells (Top) and passage 13 HFFs (Bottom) were transfected with 80 nM (total) scrambled control microRNA (CTRL), miR-181b, or miR-29a and/or miR-30e. Three days later, levels of endogenous B-Myb mRNA were measured by qRT-PCR. Two-tailed _t_-test results are indicated by * for P < 0.05 and ** for P < 0.01, relative to cells transfected with CTRL microRNA. Similar results were obtained in three (Top) or two (Bottom) independent experiments. (B) Expression of endogenous B-Myb protein was detected by Western blotting 6 d after transfection of HeLa/E6 cells with scrambled control microRNA (CTRL), miR-29a, or miR-30e, as described in A. Similar results were obtained in two independent experiments.
Fig. 6.
Interference with miR-29 and miR-30 inhibits senescence. (A) HeLa/E6 cells were transfected with 80 nM scrambled anti-miR (CTRL) or anti-miR-29a and/or anti-miR-30a inhibitors on day 0 and day 4. Thirty and 55 h after the initial transfection, cells were mock-infected or infected with Pava1 at multiplicity of infection (moi) of 15, as indicated. (A). Cells were counted 14 d after the initial transfection of anti-miRs as described above. All cells in this panel were infected with Pava1. Similar results were obtained in three independent experiments. (B). Flow cytometry was used to determine the fraction of cells proliferating after treatment with anti-miRs and E2, as indicated. The graph shows average results (+/- standard deviation) of two replicate plates of cells for each condition. Two-tailed _t_-test results are indicated by * for P < 0.05 and ** for P < 0.01, relative to cells transfected with control anti-miR and infected with Pava1. Similar results were obtained in two independent experiments.
Comment in
- MicroRNAs and senescence.
Martinez I, Almstead LL, DiMaio D. Martinez I, et al. Aging (Albany NY). 2011 Feb;3(2):77-8. doi: 10.18632/aging.100282. Aging (Albany NY). 2011. PMID: 21304181 Free PMC article. No abstract available.
Similar articles
- miR-449a causes Rb-dependent cell cycle arrest and senescence in prostate cancer cells.
Noonan EJ, Place RF, Basak S, Pookot D, Li LC. Noonan EJ, et al. Oncotarget. 2010 Sep;1(5):349-58. doi: 10.18632/oncotarget.167. Oncotarget. 2010. PMID: 20948989 Free PMC article. - The chicken miR-150 targets the avian orthologue of the functional zebrafish MYB 3'UTR target site.
Guillon-Munos A, Dambrine G, Richerioux N, Coupeau D, Muylkens B, Rasschaert D. Guillon-Munos A, et al. BMC Mol Biol. 2010 Sep 2;11:67. doi: 10.1186/1471-2199-11-67. BMC Mol Biol. 2010. PMID: 20813039 Free PMC article. - The proto-oncogene LRF is under post-transcriptional control of MiR-20a: implications for senescence.
Poliseno L, Pitto L, Simili M, Mariani L, Riccardi L, Ciucci A, Rizzo M, Evangelista M, Mercatanti A, Pandolfi PP, Rainaldi G. Poliseno L, et al. PLoS One. 2008 Jul 2;3(7):e2542. doi: 10.1371/journal.pone.0002542. PLoS One. 2008. PMID: 18596985 Free PMC article. - B-Myb, cancer, senescence, and microRNAs.
Martinez I, Dimaio D. Martinez I, et al. Cancer Res. 2011 Aug 15;71(16):5370-3. doi: 10.1158/0008-5472.CAN-11-1044. Epub 2011 Aug 9. Cancer Res. 2011. PMID: 21828240 Free PMC article. Review. - miR-20a and miR-290, multi-faceted players with a role in tumourigenesis and senescence.
Rizzo M, Mariani L, Pitto L, Rainaldi G, Simili M. Rizzo M, et al. J Cell Mol Med. 2010 Nov;14(11):2633-40. doi: 10.1111/j.1582-4934.2010.01173.x. J Cell Mol Med. 2010. PMID: 21114763 Free PMC article. Review.
Cited by
- A microRNA network regulates proliferative timing and extracellular matrix synthesis during cellular quiescence in fibroblasts.
Suh EJ, Remillard MY, Legesse-Miller A, Johnson EL, Lemons JM, Chapman TR, Forman JJ, Kojima M, Silberman ES, Coller HA. Suh EJ, et al. Genome Biol. 2012 Dec 22;13(12):R121. doi: 10.1186/gb-2012-13-12-r121. Genome Biol. 2012. PMID: 23259597 Free PMC article. - Discovery of MicroRNAs associated with myogenesis by deep sequencing of serial developmental skeletal muscles in pigs.
Hou X, Tang Z, Liu H, Wang N, Ju H, Li K. Hou X, et al. PLoS One. 2012;7(12):e52123. doi: 10.1371/journal.pone.0052123. Epub 2012 Dec 21. PLoS One. 2012. PMID: 23284895 Free PMC article. - Non-coding RNAs and their bioengineering applications for neurological diseases.
Das T, Das TK, Khodarkovskaya A, Dash S. Das T, et al. Bioengineered. 2021 Dec;12(2):11675-11698. doi: 10.1080/21655979.2021.2003667. Bioengineered. 2021. PMID: 34756133 Free PMC article. Review. - Identification of tumor suppressor miRNAs by integrative miRNA and mRNA sequencing of matched tumor-normal samples in lung adenocarcinoma.
Yu N, Yong S, Kim HK, Choi YL, Jung Y, Kim D, Seo J, Lee YE, Baek D, Lee J, Lee S, Lee JE, Kim J, Kim J, Lee S. Yu N, et al. Mol Oncol. 2019 Jun;13(6):1356-1368. doi: 10.1002/1878-0261.12478. Epub 2019 Apr 18. Mol Oncol. 2019. PMID: 30913346 Free PMC article. - Identification of nuclear-enriched miRNAs during mouse granulopoiesis.
Wong JJ, Ritchie W, Gao D, Lau KA, Gonzalez M, Choudhary A, Taft RJ, Rasko JE, Holst J. Wong JJ, et al. J Hematol Oncol. 2014 May 15;7:42. doi: 10.1186/1756-8722-7-42. J Hematol Oncol. 2014. PMID: 24886830 Free PMC article.
References
- Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
- Lee BY, et al. Senescence-associated β-galactosidase is lysosomal acid β-galactosidase. Aging Cell. 2006;5:187–195. - PubMed
- Hara E, Tsuri H, Shinozaki S, Oda K. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of lifespan in human diploid fibroblasts. Biochem Biophys Res Comm. 1991;179:528–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 CA136128/CA/NCI NIH HHS/United States
- CA16038/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- CA136128/CA/NCI NIH HHS/United States
- P01 CA016038/CA/NCI NIH HHS/United States
- T32 GM008753/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources