PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways - PubMed (original) (raw)
. 2010 Dec;30(12):4951-8.
Affiliations
- PMID: 21187475
- PMCID: PMC3141822
PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways
Roberto Gedaly et al. Anticancer Res. 2010 Dec.
Abstract
Background: Aberrant Ras/Raf/MAPK and PI3K/AKT/mTOR signaling pathways are found in hepatocellular carcinoma (HCC). This study reports how sorafenib (a multi-kinase inhibitor) and PI-103 (a dual PI3K/mTOR inhibitor) alone and in combination inhibit the proliferation of the HCC cell line, Huh7.
Materials and methods: Huh7 proliferation was assayed by 3H-thymidine incorporation and by MTT assay. Western blot was used to detect phosphorylation of the key enzymes in the Ras/Raf and PI3K pathways.
Results: Sorafenib and PI-103, as single agents inhibited Huh7 proliferation and epidermal growth factor (EGF)-stimulated Huh7 proliferation in a dose-dependent fashion; the combination of sorafenib and PI-103 produced synergistic effects. EGF increased phosphorylation of MEK and ERK, key Ras/Raf downstream signaling proteins; this activation was inhibited by sorafenib. However, sorafenib as a single agent increased AKT(Ser473) and mTOR phosphorylation. EGF-stimulated activation of PI3K/AKT/mTOR pathway components was inhibited by PI-103. PI-103 is a potent inhibitor of AKT(Ser473) phosphorylation; in contrast, rapamycin stimulated AKT(Ser473) phosphorylation. It was found that PI-103, as a single agent, stimulated MEK and ERK phosphorylation. However, the combination of sorafenib and PI-103 caused inhibition of all the tested kinases in the Ras/Raf and PI3K pathways.
Conclusion: The combination of sorafenib and PI-103 can significantly inhibit EGF-stimulated Huh7 proliferation by blocking both Ras/Raf/MAPK and PI3K/AKT/mTOR pathways.
Figures
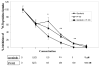
Figure 1. Effects of Sorafenib and PI-103 on inhibition of 3H-thymidine incorporation in Huh7 cells
The Huh7 cells were plated to 96 well plates at 1000 cells/well in 0.2 ml DMEM +10% FBS with quadruplicate repeats (n=4). The cells were treated with various concentrations of the drugs (as indicated) and cultured for 72h. Carrier DMSO (<0.1% final concentration) was added to the zero controls. The cells were pulsed with methyl-3H-thymidine (specificity 2 Ci/mM) for 4h at 1 μCi/well. 3H-thymidine incorporation was measured by scintillation counting in a Packard Scintillation Analyzer (model TRI-CARB 2100TR). (*: drug combination vs mono-drug, p<0.05; **: drug combination vs mono-drug, p<0.01).
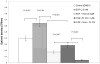
Figure 2. Sorafenib and PI-103 inhibited EGF stimulated Huh7 proliferation
(A) Cells cultured in 1% FBS starvation condition. Huh7 cells were plated in 96 well plate at 5,000 cells/well (n=12) in 100 μl of DMEM+10%FBS and cultured for 24 h. The cells were starved for 24 h in DMEM +1% FBS. Then the cells were cultured in 1% FBS in DMEM for 48h with DMSO (0.017%, control), EGF and inhibitors at the indicated concentration. MTT assay was performed as indicated in the Materials and Methods. (B) Cells cultured in 10% FBS. Huh7 cells were plated in 96 well plate at 5,000 cells/well (n=12) in 100 μl of DMEM+10% FBS and cultured for 24h. EGF and inhibitors were added for another 48 h. MTT assay was performed as indicated in the Materials and Methods.
Figure 2. Sorafenib and PI-103 inhibited EGF stimulated Huh7 proliferation
(A) Cells cultured in 1% FBS starvation condition. Huh7 cells were plated in 96 well plate at 5,000 cells/well (n=12) in 100 μl of DMEM+10%FBS and cultured for 24 h. The cells were starved for 24 h in DMEM +1% FBS. Then the cells were cultured in 1% FBS in DMEM for 48h with DMSO (0.017%, control), EGF and inhibitors at the indicated concentration. MTT assay was performed as indicated in the Materials and Methods. (B) Cells cultured in 10% FBS. Huh7 cells were plated in 96 well plate at 5,000 cells/well (n=12) in 100 μl of DMEM+10% FBS and cultured for 24h. EGF and inhibitors were added for another 48 h. MTT assay was performed as indicated in the Materials and Methods.
Figure 3
Sorafenib and PI-103 differentially inhibited or activated phosphorylation of several key enzymes in the Ras/Raf/MAPK and PI3K/Akt/mTOR pathways. (A) Western blot for p-mTOR (Ser2448), p-S6K (Thr389), p-AKT (Ser473), p-MEK1/2(Ser217/221) and p-ERK1/2(Thr202/204) level after different treatments. Also shown are endogenous mTOR, S6K, AKT, MEK1/2 and ERK1/2. Positive and negative controls for p-MEK and for p-ERK were ordered from Cell Signaling Technology (Danvers, MA) and included in the experiments but are not shown. Phosphorylated kinase band densities were assessed by Scion Image software and normalized by β-actin. (B) A plot generated based on β-actin. inormalized phosphorylated kinase band densities in Figure 4A.
Figure 3
Sorafenib and PI-103 differentially inhibited or activated phosphorylation of several key enzymes in the Ras/Raf/MAPK and PI3K/Akt/mTOR pathways. (A) Western blot for p-mTOR (Ser2448), p-S6K (Thr389), p-AKT (Ser473), p-MEK1/2(Ser217/221) and p-ERK1/2(Thr202/204) level after different treatments. Also shown are endogenous mTOR, S6K, AKT, MEK1/2 and ERK1/2. Positive and negative controls for p-MEK and for p-ERK were ordered from Cell Signaling Technology (Danvers, MA) and included in the experiments but are not shown. Phosphorylated kinase band densities were assessed by Scion Image software and normalized by β-actin. (B) A plot generated based on β-actin. inormalized phosphorylated kinase band densities in Figure 4A.
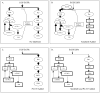
Figure 4
Possible mechanisms that sorafenib and PI-103 differentially inhibits and activates key kinases in the Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. (A) In the absence of added inhibitors, EGF/EGFR signaling activates Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Activation of S6K in the PI3K/AKT/mTOR pathway may induce feedback inhibition on Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. (B) When sorafenib is added, sorafenib directly or indirectly inhibits Raf, MEK and ERK (shown by rectangles). Sorafenib may activate HGF/HGFR signaling via activation of PI3K, thus further activating AKT, mTOC1 and mTORC2 and S6K based on the activated levels after EGF stimulation (shown by ovals). Activation of S6K still causes feedback inhibition on both pathways. (C) Addition of PI-103 directly inhibits PI3K, mTORC1 and mTORC2. PI-103 indirectly inhibits AKT and S6K in the PI3K/AKT/mTOR pathway (shown by rectangles). Inhibition of S6K eliminates the feedback inhibition effect of S6K, thus increasing the signal towards the Ras/Raf/MAPK pathway and further stimulated the Ras, Raf, MEK and ERK phosphorylation based on the activated levels after EGF stimulation (shown by ovals). (D) Combination of sorafenib and PI-103 blocks EGF stimulated Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Therefore the combination of sorafenib and PI-103 may have an advantage over mono-drug treatment in HCC therapy.
Similar articles
- Single Agent and Synergistic Activity of the "First-in-Class" Dual PI3K/BRD4 Inhibitor SF1126 with Sorafenib in Hepatocellular Carcinoma.
Singh AR, Joshi S, Burgoyne AM, Sicklick JK, Ikeda S, Kono Y, Garlich JR, Morales GA, Durden DL. Singh AR, et al. Mol Cancer Ther. 2016 Nov;15(11):2553-2562. doi: 10.1158/1535-7163.MCT-15-0976. Epub 2016 Aug 5. Mol Cancer Ther. 2016. PMID: 27496136 Free PMC article. - PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation.
Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. Gedaly R, et al. J Surg Res. 2012 Aug;176(2):542-8. doi: 10.1016/j.jss.2011.10.045. Epub 2011 Nov 21. J Surg Res. 2012. PMID: 22261591 - The role of PI3K/mTOR inhibition in combination with sorafenib in hepatocellular carcinoma treatment.
Gedaly R, Angulo P, Chen C, Creasy KT, Spear BT, Hundley J, Daily MF, Shah M, Evers BM. Gedaly R, et al. Anticancer Res. 2012 Jul;32(7):2531-6. Anticancer Res. 2012. PMID: 22753710 - Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health.
Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Milella M, Tafuri A, Bonati A, Evangelisti C, Cocco L, Martelli AM, McCubrey JA. Chappell WH, et al. Oncotarget. 2011 Mar;2(3):135-64. doi: 10.18632/oncotarget.240. Oncotarget. 2011. PMID: 21411864 Free PMC article. Review. - PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives.
Asati V, Mahapatra DK, Bharti SK. Asati V, et al. Eur J Med Chem. 2016 Feb 15;109:314-41. doi: 10.1016/j.ejmech.2016.01.012. Epub 2016 Jan 12. Eur J Med Chem. 2016. PMID: 26807863 Review.
Cited by
- Interaction of key pathways in sorafenib-treated hepatocellular carcinoma based on a PCR-array.
Liu Y, Wang P, Li S, Yin L, Shen H, Liu R. Liu Y, et al. Int J Clin Exp Pathol. 2015 Mar 1;8(3):3027-35. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045814 Free PMC article. - CDCA8 as an independent predictor for a poor prognosis in liver cancer.
Shuai Y, Fan E, Zhong Q, Chen Q, Feng G, Gou X, Zhang G. Shuai Y, et al. Cancer Cell Int. 2021 Mar 8;21(1):159. doi: 10.1186/s12935-021-01850-x. Cancer Cell Int. 2021. PMID: 33685433 Free PMC article. - Single Agent and Synergistic Activity of the "First-in-Class" Dual PI3K/BRD4 Inhibitor SF1126 with Sorafenib in Hepatocellular Carcinoma.
Singh AR, Joshi S, Burgoyne AM, Sicklick JK, Ikeda S, Kono Y, Garlich JR, Morales GA, Durden DL. Singh AR, et al. Mol Cancer Ther. 2016 Nov;15(11):2553-2562. doi: 10.1158/1535-7163.MCT-15-0976. Epub 2016 Aug 5. Mol Cancer Ther. 2016. PMID: 27496136 Free PMC article. - Dual Targeting of Sorafenib-Resistant HCC-Derived Cancer Stem Cells.
Shrestha R, Bridle KR, Cao L, Crawford DHG, Jayachandran A. Shrestha R, et al. Curr Oncol. 2021 Jun 11;28(3):2150-2172. doi: 10.3390/curroncol28030200. Curr Oncol. 2021. PMID: 34208001 Free PMC article. - Effects of RAF inhibitors on PI3K/AKT signalling depend on mutational status of the RAS/RAF signalling axis.
Fritsche-Guenther R, Witzel F, Kempa S, Brummer T, Sers C, Blüthgen N. Fritsche-Guenther R, et al. Oncotarget. 2016 Feb 16;7(7):7960-9. doi: 10.18632/oncotarget.6959. Oncotarget. 2016. PMID: 26799289 Free PMC article.
References
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
- Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752–1764. - PubMed
- Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc ) 2005;41:773–784. - PubMed
- Minguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009;25:186–194. - PubMed
- Chaparro M, Gonzalez ML, Trapero-Marugan M, Medina J, Moreno-Otero R. Review article: pharmacological therapy for hepatocellular carcinoma with sorafenib and other oral agents. Aliment Pharmacol Ther. 2008;28:1269–1277. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous