Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress - PubMed (original) (raw)
Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress
Saba Naqvi et al. Int J Nanomedicine. 2010.
Retraction in
- Concentration-Dependent Toxicity of Iron Oxide Nanoparticles Mediated by Increased Oxidative Stress [Retraction].
[No authors listed] [No authors listed] Int J Nanomedicine. 2022 Mar 25;17:1459-1460. doi: 10.2147/IJN.S367448. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35378879 Free PMC article.
Abstract
Iron oxide nanoparticles with unique magnetic properties have a high potential for use in several biomedical, bioengineering and in vivo applications, including tissue repair, magnetic resonance imaging, immunoassay, drug delivery, detoxification of biologic fluids, cell sorting, and hyperthermia. Although various surface modifications are being done for making these nonbiodegradable nanoparticles more biocompatible, their toxic potential is still a major concern. The current in vitro study of the interaction of superparamagnetic iron oxide nanoparticles of mean diameter 30 nm coated with Tween 80 and murine macrophage (J774) cells was undertaken to evaluate the dose- and time-dependent toxic potential, as well as investigate the role of oxidative stress in the toxicity. A 15-30 nm size range of spherical nanoparticles were characterized by transmission electron microscopy and zeta sizer. MTT assay showed >95% viability of cells in lower concentrations (25-200 μg/mL) and up to three hours of exposure, whereas at higher concentrations (300-500 μg/mL) and prolonged (six hours) exposure viability reduced to 55%-65%. Necrosis-apoptosis assay by propidium iodide and Hoechst-33342 staining revealed loss of the majority of the cells by apoptosis. H₂DCFDDA assay to quantify generation of intracellular reactive oxygen species (ROS) indicated that exposure to a higher concentration of nanoparticles resulted in enhanced ROS generation, leading to cell injury and death. The cell membrane injury induced by nanoparticles studied using the lactate dehydrogenase assay, showed both concentration- and time-dependent damage. Thus, this study concluded that use of a low optimum concentration of superparamagnetic iron oxide nanoparticles is important for avoidance of oxidative stress-induced cell injury and death.
Keywords: J774 cell line; MTT assay; cytotoxicity; superparamagnetic iron oxide nanoparticles.
Figures
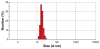
Figure 1
Zeta sizer picture of superparamagnetic iron oxide nanoparticles showing size distribution in aqueous medium.
Figure 2
Transmission electron microscopy of superparamagnetic iron oxide nanoparticles.
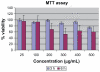
Figure 3
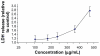
The effects of superparamagnetic iron oxide nanoparticles on cell proliferation and viability of J774 cells as determined by MTT assay. Concentration-dependent cytotoxic effects of nanoparticles evaluated after three and six hours of incubation. Results are represented as means ± standard error of the mean. **Note: ***Significant difference from control (P < 0.05).
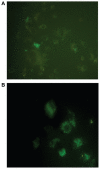
Figure 4
H2DCFDDA assay for intracellular reactive oxygen species with superparamagnetic iron oxide nanoparticles. A) Control and B) at concentration of 500 μg/mL.
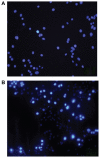
Figure 5
Apoptosis of J774 cells incubated with 500 μg/mL superparamagnetic iron oxide nanoparticles. A) Control and B) at six hours. The bright blue nuclei represent apoptosis stained with fluorescent dye Hoechst-33342.
Figure 6
Concentration-dependent membrane damage as determined by lactate dehydrogenase leakage from J774 cell lines (2 × 104 cells/mL) incubated with superparamagnetic iron oxide nanoparticles for six hours.
Figure 7
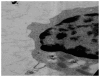
Transmission electron microscopy photograph of J774 cells showing superparamagnetic iron oxide nanoparticles in the cytosol as electron-dense particles following incubation for six hours with 200 μg/mL × 80,000.
Similar articles
- Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups.
Hong SC, Lee JH, Lee J, Kim HY, Park JY, Cho J, Lee J, Han DW. Hong SC, et al. Int J Nanomedicine. 2011;6:3219-31. doi: 10.2147/IJN.S26355. Epub 2011 Dec 7. Int J Nanomedicine. 2011. PMID: 22238510 Free PMC article. - A comparative study on the in vitro cytotoxic responses of two mammalian cell types to fullerenes, carbon nanotubes and iron oxide nanoparticles.
Dönmez Güngüneş Ç, Şeker Ş, Elçin AE, Elçin YM. Dönmez Güngüneş Ç, et al. Drug Chem Toxicol. 2017 Apr;40(2):215-227. doi: 10.1080/01480545.2016.1199563. Epub 2016 Jul 18. Drug Chem Toxicol. 2017. PMID: 27424666 - Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues.
Vakili-Ghartavol R, Momtazi-Borojeni AA, Vakili-Ghartavol Z, Aiyelabegan HT, Jaafari MR, Rezayat SM, Arbabi Bidgoli S. Vakili-Ghartavol R, et al. Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):443-451. doi: 10.1080/21691401.2019.1709855. Artif Cells Nanomed Biotechnol. 2020. PMID: 32024389 Review. - Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review.
Malhotra N, Lee JS, Liman RAD, Ruallo JMS, Villaflores OB, Ger TR, Hsiao CD. Malhotra N, et al. Molecules. 2020 Jul 10;25(14):3159. doi: 10.3390/molecules25143159. Molecules. 2020. PMID: 32664325 Free PMC article. Review.
Cited by
- Size- and age-dependent neurotoxicity of engineered metal nanoparticles in rats.
Sharma A, Muresanu DF, Patnaik R, Sharma HS. Sharma A, et al. Mol Neurobiol. 2013 Oct;48(2):386-96. doi: 10.1007/s12035-013-8500-0. Epub 2013 Jul 3. Mol Neurobiol. 2013. PMID: 23821031 - Recent Advances in the Use of Iron-Gold Hybrid Nanoparticles for Biomedical Applications.
Tarkistani MAM, Komalla V, Kayser V. Tarkistani MAM, et al. Nanomaterials (Basel). 2021 May 6;11(5):1227. doi: 10.3390/nano11051227. Nanomaterials (Basel). 2021. PMID: 34066549 Free PMC article. Review. - The exposure of cancer cells to hyperthermia, iron oxide nanoparticles, and mitomycin C influences membrane multidrug resistance protein expression levels.
Franke K, Kettering M, Lange K, Kaiser WA, Hilger I. Franke K, et al. Int J Nanomedicine. 2013;8:351-63. doi: 10.2147/IJN.S37465. Epub 2013 Jan 20. Int J Nanomedicine. 2013. PMID: 23378758 Free PMC article. - Novel Strategies for Disrupting Cancer-Cell Functions with Mitochondria-Targeted Antitumor Drug-Loaded Nanoformulations.
S Allemailem K, Almatroudi A, Alsahli MA, Aljaghwani A, M El-Kady A, Rahmani AH, Khan AA. S Allemailem K, et al. Int J Nanomedicine. 2021 Jun 9;16:3907-3936. doi: 10.2147/IJN.S303832. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34135584 Free PMC article. Review. - Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects.
Negrescu AM, Killian MS, Raghu SNV, Schmuki P, Mazare A, Cimpean A. Negrescu AM, et al. J Funct Biomater. 2022 Dec 5;13(4):274. doi: 10.3390/jfb13040274. J Funct Biomater. 2022. PMID: 36547533 Free PMC article. Review.
References
- Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95(2):200–206. - PubMed
- Polyak B, Friedman G. Magnetic targeting for site-specific drug delivery: Applications and clinical potential. Expert Opin Drug Deliv. 2009;6(1):53–70. - PubMed
- Shi J, Gider S, Babcock K, et al. Magnetic clusters in molecular beams, metals, and semiconductors. Science. 1996;271:937–941.
- Sastry M. Moving nanoparticles around: Phase-transfer processes in nanomaterials synthesis. In: Rao CNR, Achim Muller HC, Cheetham AK, editors. The Chemistry of Nanomaterials. Weinheim, Germany: Wiley-VCH, Verlag GmbH and Co. KGaA; 2005.
- Lubbe AS, Bergemann C, Riess H, et al. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996;56(20):4686–4693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources