The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis - PubMed (original) (raw)
The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis
Athena L Chen et al. Front Neurol. 2010.
Abstract
Progressive supranuclear palsy (PSP) is a disease of later life that is currently regarded as a form of neurodegenerative tauopathy. Disturbance of gaze is a cardinal clinical feature of PSP that often helps clinicians to establish the diagnosis. Since the neurobiology of gaze control is now well understood, it is possible to use eye movements as investigational tools to understand aspects of the pathogenesis of PSP. In this review, we summarize each disorder of gaze control that occurs in PSP, drawing on our studies of 50 patients, and on reports from other laboratories that have measured the disturbances of eye movements. When these gaze disorders are approached by considering each functional class of eye movements and its neurobiological basis, a distinct pattern of eye movement deficits emerges that provides insight into the pathogenesis of PSP. Although some aspects of all forms of eye movements are affected in PSP, the predominant defects concern vertical saccades (slow and hypometric, both up and down), impaired vergence, and inability to modulate the linear vestibulo-ocular reflex appropriately for viewing distance. These vertical and vergence eye movements habitually work in concert to enable visuomotor skills that are important during locomotion with the hands free. Taken with the prominent early feature of falls, these findings suggest that PSP tauopathy impairs a recently evolved neural system concerned with bipedal locomotion in an erect posture and frequent gaze shifts between the distant environment and proximate hands. This approach provides a conceptual framework that can be used to address the nosological challenge posed by overlapping clinical and neuropathological features of neurodegenerative tauopathies.
Keywords: parkinsonian disorders; saccades; tauopathy; vergence; vestibular.
Figures
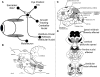
Figure 1
(A) Schematic summary of main premotor inputs to motoneurons innervating vertically acting extraocular muscles. riMLF, rostral interstitial nucleus of the medial longitudinal fasciculus; INC, interstitial nucleus of Cajal. See text for commentary. (B) Sagittal section of the monkey brain stem showing the locations of premotor burst neurons: excitatory burst neurons (EBN) for horizontal saccades lie in the paramedian pontine reticular formation (PPRF); inhibitory burst neurons (IBN) for horizontal saccades lie in the medullary reticular formation (Med RF); EBN for vertical and torsional saccades lie in the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF). Some vertical IBN may reside in or close to the interstitial nucleus of Cajal (INC). EBN and IBN project to ocular motoneurons lying in the abducens nucleus (VI), the trochlear nucleus (IV) and the oculomotor nucleus (III). Omnipause neurons (indicated by an asterisk) lie in the midline raphe of the pons between the rootlets of the abducens nerve (CN VI) and gate the activity of EBN and IBN. The mesencephalic reticular formation (MRF) may also contribute to vertical saccades. CG, central gray; MB, mammillary body; CN III, rootlets of the oculomotor nerve; CN IV, trochlear nerve; ND, nucleus of Darkschewitsch; NRTP, nucleus reticularis tegmenti pontis; PC, posterior commissure; NPH, nucleus prepositus hypoglossi; sc, superior colliculus; TR, tractus retroflexus. The arrow refers to the Horsley–Clarke plane of section. Figure courtesy of Dr. Jean Büttner-Ennever. (C) Probable location of cortical areas important for eye movements in human brain. MST, medial superior temporal visual area; MT, middle temporal visual area. Adapted from Leigh and Zee (2006) with permission. (D) Figures adapted with permission from Steele et al. (1964) that summarize the brainstem regions affected in PSP; the intensity of shading corresponds to the severity of the involvement.
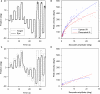
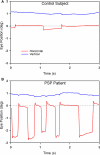
Figure 2
Vertical saccades made by a normal subject and a patient with PSP. (A) Representative healthy elderly subject's target-directed vertical saccades. (B) Peak velocity–amplitude relationship of these saccades, as modeled by the equation (peak velocity = K × amplitude_L_). Both fit curves have _R_2 > 0.9. This particular subject made faster upward saccades than downward saccades. (C) PSP patient's saccades to similar target jumps as for the control in (A). (D) PSP patient's peak velocity–amplitude fit curves; both have _R_2 > 0.75. The curve fits for this PSP patient's upward and downward saccades show overlap.
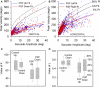
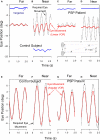
Figure 3
Peak velocity–amplitude relationship of vertical saccades (A) and horizontal saccades (B) made by control subjects (CS) and PSP patients. (A) Upward and downward power fits calculated for all vertical saccades made by 17 PSP patients for whom _R_2 > 0.7. Fit, 5 and 95% prediction interval (PI) for all CS saccades are shown. Most of the faster downward saccades were made by one patient with 1.5 year disease duration. (B) Horizontal saccades made by PSP patients were slower than those made by CS (with no left-right asymmetry), but slowing was less marked than for vertical saccades. (C) Box-plot comparison of _K_-values for controls’ and patients’ vertical saccade power fits. Intragroup differences are not significant, but the difference between controls’ _K_-values and patients’ _K_-values is (p < 0.001). (D) Comparison of _L_-values for controls’ and patients’ vertical saccade power fits. Only the intergroup L was found to be different (p = 0.013). Box-plot percentiles are shown at right.
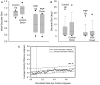
Figure 4
Comparison of size of vertical saccades made by controls and PSP patients. (A) Gain comparison. Downward gain was larger than upward gain for our 10 control subjects (p = 0.006), but there was no difference for the 10 PSP patients tested. (B) Amplitude comparison. Downward saccade size was larger than the upward saccade size for both the elderly control (p < 0.001) and PSP patient (p = 0.002) groups. Box-plot symbols are similar to Figure 3; dot symbols represent 5 and 95% percentiles. (C) Conjugacy of vertical saccades made by PSP patients using the approach of binocular phase planes. The abscissa scale is normalized eye displacement. The ordinate scale is the difference in normalized eye speed between the two eyes. The dashed lines are 5 and 95% prediction intervals (PI) based on over a thousand saccades made by 10 normal subjects. Although PSP patients made slower vertical saccades than control subjects, their eye movements are tightly conjugate.
Figure 5
Comparison of fixation behavior in a normal elderly subject (A) and in a patient with PSP (B). Both show saccadic intrusions (square-wave jerks), but they are larger and more frequent in the record from the PSP patient. The gray dashed line corresponds to the desired horizontal position of fixation. The vertical channel has been offset in both records to aid clarity. Positive values indicate rightward and upward movements.
Figure 6
Comparison of the vertical linear or translational (bob) vestibulo-ocular reflex (tVOR) in the top panels and the horizontal angular (yaw) vestibulo-ocular reflex (aVOR) in the lower panels from a normal elderly subject (A,B,E,F) and a patient with PSP (C,D,G,H). When the normal subject viewed the far target (A), vertical eye movements (red line) due to tVOR were appropriately small; during near viewing (B), when the subject converged (blue line), they increase, although not as much as would be required to hold the foveal line of sight on the visual target (black dotted line). When the PSP patient subject viewed the far target (C), vertical eye movements due to tVOR were small; during near viewing (D), the patient neither converged nor increased the tVOR response. The normal subject shows near-perfect aVOR during far viewing (E); during near viewing (F), the magnitude of the response increases by about 24%, although not as much as needed for ideal viewing. The PSP patient also shows a near-perfect aVOR during far viewing (G); however, during near viewing (H) there is no measurable increase of the response, as is required by geometric factors (see text). Note that positive values indicate downward, leftward or divergence movements in this figure; different records have been offset to aid clarity except for vergence. The angle of vergence achieved in each of the lower panels corresponds to that in the panel above. The inset in the lower right corner of the upper panel summarizes the geometric relationship between viewing distance (D), amplitude of head translation (A), and the angle of eye rotation (θ) required to hold the foveal line of sight on the target.
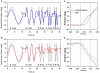
Figure 7
Comparison of vertical smooth pursuit (A,B) and ocular following responses (C,D) of a normal subject and a PSP patient. Both sets of responses were evoked as subjects viewed vertically-moving sine-wave gratings on a monitor subtending 50° × 37.5°. For pursuit, the stimulus was sinusoidal motion of a 0.27 cycles per degree grating over the range of temporal frequencies shown. For OFR, the stimulus was ramp motion for 200 ms of a 0.17 cycles per degree grating. At lower frequencies of motion, both the normal subject (A) and the PSP patient (B) showed smooth tracking with some predictive properties (arrows); at high frequencies, the PSP (B) showed substantial decrease in the size of the pursuit movements compared with the control subject. Both upward (C) and downward (D) grating motion induced similar-sized OFR from the control subject (blue line) and PSP patient (red line), although the latency to onset was larger in the patient. See Joshi et al. (2010) for details.
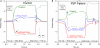
Figure 8
Comparison of combined saccade–vergence responses as a control subject (A) or a patient with early PSP (B) attempted to make shifts of the point of fixation between two visual targets aligned on the left eye (Müller paradigm), one located at far and one at near [see inset in (A)]; the far target was higher than the near target, requiring an associated vertical saccade. The normal subject uses disjunctive saccades (right movement bigger than left) followed by a vergence movement (arrows) for shifts of the point of fixation in both directions. The PSP patient could not generate an adequate vergence movement (compare with control subject; asterisk signifies a superimposed blink). Furthermore, the horizontal saccadic components were more similar in size (less disjunctive than the control). The associated vertical saccade is also slower in the PSP patient. Thus, the complete synkinesis of saccades and vergence is affected in this PSP patient.
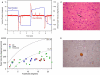
Figure 9
Summary of findings from Patient 1 with pure akinesia. (A) Vertical saccades 14 years after the onset of his illness showed modest slowing and hypometria. (B) Comparison of peak velocity of vertical and horizontal saccades with 10 age-matched normal subjects (for whom 5 and 95% confidence intervals are shown by dashed lines). Larger saccades made by Patient 1 in all directions are slowed compared with controls. (C) Microscopic findings at autopsy showed numerous globose neurofibrillary tangles (NFTs) in the basal ganglia, including STN (example indicated by arrow). (D) A pontine NFT demonstrating marked labeling by anti-tau immunohistochemistry. See text for details.
Similar articles
- Functional correlates of vertical gaze palsy and other ocular motor deficits in PSP: an FDG-PET study.
Amtage F, Maurer C, Hellwig S, Tüscher O, Kreft A, Weiller C, Rijntjes M, Winkler C, Meyer PT. Amtage F, et al. Parkinsonism Relat Disord. 2014 Aug;20(8):898-906. doi: 10.1016/j.parkreldis.2014.05.013. Epub 2014 Jun 4. Parkinsonism Relat Disord. 2014. PMID: 24935235 - Video-oculographic biomarkers for evaluating vertical ocular dysfunction in progressive supranuclear palsy.
Quattrone A, Crasà M, Morelli M, Vescio B, Augimeri A, Gramigna V, Quattrone A. Quattrone A, et al. Parkinsonism Relat Disord. 2022 Jun;99:84-90. doi: 10.1016/j.parkreldis.2022.05.014. Epub 2022 May 22. Parkinsonism Relat Disord. 2022. PMID: 35642995 - Case report: Saccadic ping-pong gaze in progressive supranuclear palsy with predominant postural instability.
Nunomura H, Kasahara T, Hatano T, Shimada H, Takado Y, Endo H, Inoshita A, Inomata A, Murofushi T, Misawa S, Machida Y, Imai H. Nunomura H, et al. Front Neurol. 2023 Mar 1;14:1100931. doi: 10.3389/fneur.2023.1100931. eCollection 2023. Front Neurol. 2023. PMID: 36937509 Free PMC article. - Central ocular motor disorders, including gaze palsy and nystagmus.
Strupp M, Kremmyda O, Adamczyk C, Böttcher N, Muth C, Yip CW, Bremova T. Strupp M, et al. J Neurol. 2014 Sep;261 Suppl 2(Suppl 2):S542-58. doi: 10.1007/s00415-014-7385-9. J Neurol. 2014. PMID: 25145891 Free PMC article. Review. - The linear vestibulo-ocular reflex, locomotion and falls in neurological disorders.
Liao K, Walker MF, Joshi AC, Reschke M, Strupp M, Wagner J, Leigh RJ. Liao K, et al. Restor Neurol Neurosci. 2010;28(1):91-103. doi: 10.3233/RNN-2010-0507. Restor Neurol Neurosci. 2010. PMID: 20086286 Review.
Cited by
- Reduced maximal range of ocular movements and its response to acute levodopa challenge in Parkinson's disease.
Li J, Li Y, Chu X, Jiang M, Wu T, Chen X. Li J, et al. Front Aging Neurosci. 2024 Mar 20;16:1368539. doi: 10.3389/fnagi.2024.1368539. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38572152 Free PMC article. - Ocular Tremor in Parkinson's Disease: Discussion, Debate, and Controversy.
Kaski D, Bronstein AM. Kaski D, et al. Front Neurol. 2017 Apr 24;8:134. doi: 10.3389/fneur.2017.00134. eCollection 2017. Front Neurol. 2017. PMID: 28484420 Free PMC article. Review. - Intrinsic connectivity network disruption in progressive supranuclear palsy.
Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, Heuer HW, Fine E, Zhou J, Kramer JH, Miller BL, Seeley WW. Gardner RC, et al. Ann Neurol. 2013 May;73(5):603-16. doi: 10.1002/ana.23844. Epub 2013 Mar 27. Ann Neurol. 2013. PMID: 23536287 Free PMC article. - Diagnostic value of video-oculography in progressive supranuclear palsy: a controlled study in 100 patients.
Wunderlich J, Behler A, Dreyhaupt J, Ludolph AC, Pinkhardt EH, Kassubek J. Wunderlich J, et al. J Neurol. 2021 Sep;268(9):3467-3475. doi: 10.1007/s00415-021-10522-9. Epub 2021 Mar 21. J Neurol. 2021. PMID: 33744980 Free PMC article. - Anti-IgLON5 Disease Showing an Improvement in Dysautonomia, Including Vocal Cord Palsy, via Combined Immunotherapy.
Sato D, Sato H, Kondo T, Igari R, Iseki C, Kawahara H, Amano S, Ono Y, Kimura A, Shimohata T, Ohta Y. Sato D, et al. Intern Med. 2024 Aug 1;63(15):2187-2191. doi: 10.2169/internalmedicine.2865-23. Epub 2024 Jan 2. Intern Med. 2024. PMID: 38171876 Free PMC article.
References
- Bhidayasiri R., Riley D. E., Somers J. T., Lerner A. J., Buttner-Ennever J. A., Leigh R. J. (2001). Pathophysiology of slow vertical saccades in progressive supranuclear palsy. Neurology 57, 2070–2077 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous