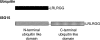Interferon-stimulated gene 15 and the protein ISGylation system - PubMed (original) (raw)
Review
Interferon-stimulated gene 15 and the protein ISGylation system
Dongxian Zhang et al. J Interferon Cytokine Res. 2011 Jan.
Abstract
Interferon-stimulated gene 15 (ISG15) is one of the most upregulated genes upon Type I interferon treatment or pathogen infection. Its 17 kDa protein product, ISG15, was the first ubiquitin-like modifier identified, and is similar to a ubiquitin linear dimer. As ISG15 modifies proteins in a similar manner to ubiquitylation, protein conjugation by ISG15 is termed ISGylation. Some of the primary enzymes that promote ISGylation are also involved in ubiquitin conjugation. The process to remove ISG15 from its conjugated proteins, termed de-ISGylation, is performed by a cellular ISG15-specific protease, ubiquitin-specific proteases with molecular mass 43 kDa (UBP43)/ubiquitin-specific proteases 18. Relative to ubiquitin, the biological function of ISG15 is still poorly understood, but ISG15 appears to play important roles in various biological and cellular functions. Therefore, there is growing interest in ISG15, as the study of free ISG15 and functional consequences of ISGylation/de-ISGylation may identify useful therapeutic targets. This review highlights recent discoveries and remaining questions important to understanding the biological functions of ISG15.
Figures
FIG. 1.
A domain diagram of ubiquitin and ISG15. ISG15, interferon-stimulated gene 15; LRLRGG, Leu Arg Leu Arg Gly Gly.
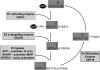
FIG. 2.
Protein ISGylation and de-ISGylaiton system. UbE1L catalyzes adenylation and then forms a thioester bond with the C-terminal end of ISG15. Activated ISG15 is transferred to the ISG15-conjugating enzyme, UbCH8. ISG15 and UbCH8 are also covalently linked via a thioester bond. With the help of ISG15 E3 ligase, the C-terminus of ISG15 is conjugated to the ɛ-amine group of lysine on the substrate. Ubiquitin-specific proteases 18 removes ISG15 from the substrate.
Similar articles
- Molecular Pathways of Interferon-Stimulated Gene 15: Implications in Cancer.
Tecalco-Cruz AC. Tecalco-Cruz AC. Curr Protein Pept Sci. 2021;22(1):19-28. doi: 10.2174/1389203721999201208200747. Curr Protein Pept Sci. 2021. PMID: 33292152 Review. - Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation.
Kim KI, Giannakopoulos NV, Virgin HW, Zhang DE. Kim KI, et al. Mol Cell Biol. 2004 Nov;24(21):9592-600. doi: 10.1128/MCB.24.21.9592-9600.2004. Mol Cell Biol. 2004. PMID: 15485925 Free PMC article. - The ISG15/USP18 ubiquitin-like pathway (ISGylation system) in hepatitis C virus infection and resistance to interferon therapy.
Chen L, Li S, McGilvray I. Chen L, et al. Int J Biochem Cell Biol. 2011 Oct;43(10):1427-31. doi: 10.1016/j.biocel.2011.06.006. Epub 2011 Jun 16. Int J Biochem Cell Biol. 2011. PMID: 21704181 Review. - A negative feedback of the HIF-1α pathway via interferon-stimulated gene 15 and ISGylation.
Yeh YH, Yang YC, Hsieh MY, Yeh YC, Li TK. Yeh YH, et al. Clin Cancer Res. 2013 Nov 1;19(21):5927-39. doi: 10.1158/1078-0432.CCR-13-0018. Epub 2013 Sep 20. Clin Cancer Res. 2013. PMID: 24056783 - An Approach for the Identification of Proteins Modified with ISG15.
Takeuchi T, Koinuma S, Yokosawa H, Arata Y. Takeuchi T, et al. Methods Mol Biol. 2019;1934:235-246. doi: 10.1007/978-1-4939-9055-9_15. Methods Mol Biol. 2019. PMID: 31256383
Cited by
- Up-regulation of interferon-stimulated gene15 and its conjugates by tumor necrosis factor-α via type I interferon-dependent and -independent pathways.
Chairatvit K, Wongnoppavich A, Choonate S. Chairatvit K, et al. Mol Cell Biochem. 2012 Sep;368(1-2):195-201. doi: 10.1007/s11010-012-1360-5. Epub 2012 Jun 23. Mol Cell Biochem. 2012. PMID: 22729740 - Inflammatory cutaneous lesions and pulmonary manifestations in a new patient with autosomal recessive ISG15 deficiency case report.
Buda G, Valdez RM, Biagioli G, Olivieri FA, Affranchino N, Bouso C, Lotersztein V, Bogunovic D, Bustamante J, Martí MA. Buda G, et al. Allergy Asthma Clin Immunol. 2020 Sep 3;16:77. doi: 10.1186/s13223-020-00473-7. eCollection 2020. Allergy Asthma Clin Immunol. 2020. PMID: 32944031 Free PMC article. - Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway.
Rojas JM, Alejo A, Martín V, Sevilla N. Rojas JM, et al. Cell Mol Life Sci. 2021 Feb;78(4):1423-1444. doi: 10.1007/s00018-020-03671-z. Epub 2020 Oct 21. Cell Mol Life Sci. 2021. PMID: 33084946 Free PMC article. Review. - USP18 directly regulates Snail1 protein through ubiquitination pathway in colorectal cancer.
Huang F, Zheng C, Huang L, Lin C, Wang J. Huang F, et al. Cancer Cell Int. 2020 Jul 28;20:346. doi: 10.1186/s12935-020-01442-1. eCollection 2020. Cancer Cell Int. 2020. PMID: 32742193 Free PMC article. - Deubiquitinating Enzymes and Bone Remodeling.
Guo YC, Zhang SW, Yuan Q. Guo YC, et al. Stem Cells Int. 2018 Jul 8;2018:3712083. doi: 10.1155/2018/3712083. eCollection 2018. Stem Cells Int. 2018. PMID: 30123285 Free PMC article. Review.
References
- Amerik AY. Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695(1–3):189–207. - PubMed
- Bektas N. Noetzel E. Veeck J. Press MF. Kristiansen G. Naami A. Hartmann A. Dimmler A. Beckmann MW. Knuchel R. Fasching PA. Dahl E. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res. 2008;10(4):R58. - PMC - PubMed
- Blomstrom DC. Fahey D. Kutny R. Korant BD. Knight E., Jr. Molecular characterization of the interferon-induced 15-kDa protein. Molecular cloning and nucleotide and amino acid sequence. J Biol Chem. 1986;261(19):8811–8816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous