Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration - PubMed (original) (raw)
Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration
Alexander K Epstein et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Most of the world's bacteria exist in robust, sessile communities known as biofilms, ubiquitously adherent to environmental surfaces from ocean floors to human teeth and notoriously resistant to antimicrobial agents. We report the surprising observation that Bacillus subtilis biofilm colonies and pellicles are extremely nonwetting, greatly surpassing the repellency of Teflon toward water and lower surface tension liquids. The biofilm surface remains nonwetting against up to 80% ethanol as well as other organic solvents and commercial biocides across a large and clinically important concentration range. We show that this property limits the penetration of antimicrobial liquids into the biofilm, severely compromising their efficacy. To highlight the mechanisms of this phenomenon, we performed experiments with mutant biofilms lacking ECM components and with functionalized polymeric replicas of biofilm microstructure. We show that the nonwetting properties are a synergistic result of ECM composition, multiscale roughness, reentrant topography, and possibly yet other factors related to the dynamic nature of the biofilm surface. Finally, we report the impenetrability of the biofilm surface by gases, implying defense capability against vapor-phase antimicrobials as well. These remarkable properties of B. subtilis biofilm, which may have evolved as a protection mechanism against native environmental threats, provide a new direction in both antimicrobial research and bioinspired liquid-repellent surface paradigms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
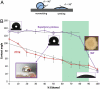
Bacterial biofilm wetting characterization by contact angle analysis. (A) Schematic of the contact angle θ: low (high) surface tension liquids generally wet (do not wet) surfaces and have small (large) contact angles. (B) Contact angle of water droplets on a WT B. subtilis biofilm and a Teflon block as a function of ethanol concentration. A plateau of ∼135–145° is seen for the biofilm up to ∼80% ethanol, when it transitions to wetting. In contrast, Teflon displays a roughly linear decrease in contact angle. Liquid drop profiles used for determining the contact angle are inset for wild-type biofilm at 0, 50, and 100% ethanol. Antimicrobial activity of alcohols is believed to be optimal in the 60 to 90% range, denoted as the green region, where the biofilm is largely nonwetting, suggesting that ethanol-based bactericides may not wick into the biofilm. Error bars are SD, n = 7. (Insets) The architecture of the wild-type biofilm (Right) and a nonwetting droplet of 50% ethanol on the biofilm surface (Left).
Fig. 2.
Fluorescent confocal z stack showing 3D rhodamine staining of B. subtilis colony integrated through the thickness of the film. (A) Failure of liquid to access wild-type colony. Areas that are stained red correspond to regions that were wetted by the puddle—the liquid footprint—and penetration is only partial. Black, unstained regions correspond to trapped air. (B) Uniform fluorescent staining of epsH colony indicates complete, uniform wetting of the entire mutant biofilm by liquid. Scale bars are 250 μm.
Fig. 3.
Persistent biofilm nonwettability is invariable with respect to ethanol concentration across the repellent concentration range. Drops of four ethanol–water concentrations were tracked for contact angle during evaporation on the surfaces of wild-type B. subtilis colonies. (A) Evaporation contact angles decay faster in time for higher ethanol concentrations. This is expected due to ethanol’s high vapor pressure and due to some contact line pinning, or sticking of the droplet edge to surface heterogeneities (16). (B) The evaporation contact angle as a function of drop volume, however, traces the same curve for deionized water, 30%, and 60% ethanol, in spite of dramatic surface tension decrease, and shows large deviation only at the 80% grade—roughly the threshold concentration between nonwetting and wetting behavior.
Fig. 4.
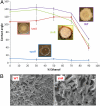
Characterization of liquid repellency mechanisms using genetic mutants of B. subtilis biofilms lacking either the carbohydrate-rich epsH or protein tasA, or sinR. (A) The phenotypes are inset adjacent to their respective contact angle curves. Highly wrinkled sinR biofilm, with excess tasA protein and epsH, exhibits slightly decreased repellency relative to wild type, possibly related to suboptimal topography. Error bars are SD, n = 7 for WT and Teflon, n = 8+ for tasA, 8+ for epsH, and 12+ for sinR. A standard Wilcoxon two-sided test was performed to test statistical significance in contact angle differences (1% and 5% significance level). The contact angle for epsH is statistically different from any other strain; WT is statistically different from tasA at all ethanol concentrations, and from sinR at ethanol concentrations ≥50%; tasA and sinR are statistically different except at 50% and 90% ethanol concentration (and 70% at significance level 1%). (B) Corresponding magnification SEM images showing the surface features of the critical point dried WT biofilm (Left) and the sinR mutant (Right).
Fig. 5.
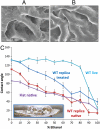
Testing role of topography using functionalized polymeric replicas of biofilm microstructure. (A) SEM image showing the surface features of the critical point dried live WT biofilm; (B) SEM image showing the surface features of the UV-cured epoxy replica of the wild-type biofilm. Microscale topography is reproduced well, although dehydration artifacts may occur in the critical point dried sample. (C) Contact angle of a live wild-type colony, a native (uncoated) epoxy replica fabricated by adapting soft lithography (18), a fluorinated replica, and a native (uncoated) flat epoxy substrate. (Inset) Epoxy biofilm replica with applied drops of 30% ethanol. Error bars are SD, n = 7.
Fig. 6.
Synchrotron microcomputed tomography reconstructed images of the (A) WT, (B) tasA mutant, and (C) epsH mutant of B. subtilis biofilms following atomic layer deposition (ALD) of heavy metal oxides by vapor exposure (see Materials and Methods). In the wild-type biofilm, the vapor-phase ALD precursors penetrate only a short distance. In tasA and particularly the eps-deficient colony, deep gas penetration was observed.
Similar articles
- BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms.
Kobayashi K, Iwano M. Kobayashi K, et al. Mol Microbiol. 2012 Jul;85(1):51-66. doi: 10.1111/j.1365-2958.2012.08094.x. Epub 2012 May 28. Mol Microbiol. 2012. PMID: 22571672 - Metal ions weaken the hydrophobicity and antibiotic resistance of Bacillus subtilis NCIB 3610 biofilms.
Falcón García C, Kretschmer M, Lozano-Andrade CN, Schönleitner M, Dragoŝ A, Kovács ÁT, Lieleg O. Falcón García C, et al. NPJ Biofilms Microbiomes. 2020 Jan 3;6:1. doi: 10.1038/s41522-019-0111-8. eCollection 2020. NPJ Biofilms Microbiomes. 2020. PMID: 31908831 Free PMC article. - Direct Comparison of Physical Properties of Bacillus subtilis NCIB 3610 and B-1 Biofilms.
Kesel S, Grumbein S, Gümperlein I, Tallawi M, Marel AK, Lieleg O, Opitz M. Kesel S, et al. Appl Environ Microbiol. 2016 Apr 4;82(8):2424-2432. doi: 10.1128/AEM.03957-15. Print 2016 Apr. Appl Environ Microbiol. 2016. PMID: 26873313 Free PMC article. - Just in case it rains: building a hydrophobic biofilm the Bacillus subtilis way.
Arnaouteli S, MacPhee CE, Stanley-Wall NR. Arnaouteli S, et al. Curr Opin Microbiol. 2016 Dec;34:7-12. doi: 10.1016/j.mib.2016.07.012. Epub 2016 Jul 25. Curr Opin Microbiol. 2016. PMID: 27458867 Review. - Evolved Biofilm: Review on the Experimental Evolution Studies of Bacillus subtilis Pellicles.
Kovács ÁT, Dragoš A. Kovács ÁT, et al. J Mol Biol. 2019 Nov 22;431(23):4749-4759. doi: 10.1016/j.jmb.2019.02.005. Epub 2019 Feb 12. J Mol Biol. 2019. PMID: 30769118 Review.
Cited by
- Synthesis and patterning of tunable multiscale materials with engineered cells.
Chen AY, Deng Z, Billings AN, Seker UO, Lu MY, Citorik RJ, Zakeri B, Lu TK. Chen AY, et al. Nat Mater. 2014 May;13(5):515-23. doi: 10.1038/nmat3912. Epub 2014 Mar 23. Nat Mater. 2014. PMID: 24658114 Free PMC article. - Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms.
Pandit S, Ravikumar V, Abdel-Haleem AM, Derouiche A, Mokkapati VRSS, Sihlbom C, Mineta K, Gojobori T, Gao X, Westerlund F, Mijakovic I. Pandit S, et al. Front Microbiol. 2017 Dec 22;8:2599. doi: 10.3389/fmicb.2017.02599. eCollection 2017. Front Microbiol. 2017. PMID: 29317857 Free PMC article. - The potency of bacteriophages isolated from chicken intestine and beef tribe to control biofilm-forming bacteria, Bacillus subtilis.
Wardani AK, Buana EOGHN, Sutrisno A. Wardani AK, et al. Sci Rep. 2023 May 22;13(1):8222. doi: 10.1038/s41598-023-35474-0. Sci Rep. 2023. PMID: 37217567 Free PMC article. - Photodynamic antimicrobial polymers for infection control.
McCoy CP, O'Neil EJ, Cowley JF, Carson L, De Baróid ÁT, Gdowski GT, Gorman SP, Jones DS. McCoy CP, et al. PLoS One. 2014 Sep 24;9(9):e108500. doi: 10.1371/journal.pone.0108500. eCollection 2014. PLoS One. 2014. PMID: 25250740 Free PMC article. - The Good, the Bad, and the Ugly: Mycotoxin Production During Postharvest Decay and Their Influence on Tritrophic Host-Pathogen-Microbe Interactions.
Bartholomew HP, Bradshaw M, Jurick WM 2nd, Fonseca JM. Bartholomew HP, et al. Front Microbiol. 2021 Feb 12;12:611881. doi: 10.3389/fmicb.2021.611881. eCollection 2021. Front Microbiol. 2021. PMID: 33643240 Free PMC article. Review.
References
- Costerton JW, Stewart PS. Battling biofilms—The war is against bacterial colonies that cause some of the most tenacious infections known. The weapon is knowledge of the enemy’s communication system. Sci Am. 2001;285(1):74–81. - PubMed
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources