Leaf-cutting ant fungi produce cell wall degrading pectinase complexes reminiscent of phytopathogenic fungi - PubMed (original) (raw)
Leaf-cutting ant fungi produce cell wall degrading pectinase complexes reminiscent of phytopathogenic fungi
Morten Schiøtt et al. BMC Biol. 2010.
Abstract
Background: Leaf-cutting (attine) ants use their own fecal material to manure fungus gardens, which consist of leaf material overgrown by hyphal threads of the basidiomycete fungus Leucocoprinus gongylophorus that lives in symbiosis with the ants. Previous studies have suggested that the fecal droplets contain proteins that are produced by the fungal symbiont to pass unharmed through the digestive system of the ants, so they can enhance new fungus garden growth.
Results: We tested this hypothesis by using proteomics methods to determine the gene sequences of fecal proteins in Acromyrmex echinatior leaf-cutting ants. Seven (21%) of the 33 identified proteins were pectinolytic enzymes that originated from the fungal symbiont and which were still active in the fecal droplets produced by the ants. We show that these enzymes are found in the fecal material only when the ants had access to fungus garden food, and we used quantitative polymerase chain reaction analysis to show that the expression of six of these enzyme genes was substantially upregulated in the fungal gongylidia. These unique structures serve as food for the ants and are produced only by the evolutionarily advanced garden symbionts of higher attine ants, but not by the fungi reared by the basal lineages of this ant clade.
Conclusions: Pectinolytic enzymes produced in the gongylidia of the fungal symbiont are ingested but not digested by Acromyrmex leaf-cutting ants so that they end up in the fecal fluid and become mixed with new garden substrate. Substantial quantities of pectinolytic enzymes are typically found in pathogenic fungi that attack live plant tissue, where they are known to breach the cell walls to allow the fungal mycelium access to the cell contents. As the leaf-cutting ant symbionts are derived from fungal clades that decompose dead plant material, our results suggest that their pectinolytic enzymes represent secondarily evolved adaptations that are convergent to those normally found in phytopathogens.
Figures
Figure 1
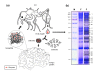
The life cycle of fecal droplet proteins. (a) Degradation enzymes produced in the gongylidia are passed through the ant alimentary system to end up in the fecal fluid, fecal droplets of which are mixed with the new plant substrate to form leaf pulp that the ants add to the fungus garden. (b) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of ant fecal fluid. M, molecular size marker. F, fecal fluid. Excised bands are indicated by numbered boxes in the right-hand version of duplicated lane F.
Figure 2
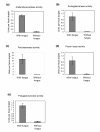
Pectinolytic enzyme activities in fecal material from ants with access to fungal symbiont food compared to ants deprived of their fungus garden material for 2 weeks. Bars are means ± SE (n = 9).
Figure 3
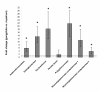
Expression levels of pectinolytic enzyme genes in gongylidia relative to gongylidia-free mycelium measured with quantitative real-time polymerase chain reaction (qPCR). Bars are means ± SE (n = 4). Statistically significant results are marked with an asterisk. An equal level of gene expression between the two tissues gives a fold change of one (dotted horizontal line).
Similar articles
- Ant mediated redistribution of a xyloglucanase enzyme in fungus gardens of Acromyrmex echinatior.
Kooij PW, Pullens JW, Boomsma JJ, Schiøtt M. Kooij PW, et al. BMC Microbiol. 2016 May 6;16:81. doi: 10.1186/s12866-016-0697-4. BMC Microbiol. 2016. PMID: 27154066 Free PMC article. - Laccase detoxification mediates the nutritional alliance between leaf-cutting ants and fungus-garden symbionts.
De Fine Licht HH, Schiøtt M, Rogowska-Wrzesinska A, Nygaard S, Roepstorff P, Boomsma JJ. De Fine Licht HH, et al. Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):583-7. doi: 10.1073/pnas.1212709110. Epub 2012 Dec 24. Proc Natl Acad Sci U S A. 2013. PMID: 23267060 Free PMC article. - The fungal symbiont of Acromyrmex leaf-cutting ants expresses the full spectrum of genes to degrade cellulose and other plant cell wall polysaccharides.
Grell MN, Linde T, Nygaard S, Nielsen KL, Boomsma JJ, Lange L. Grell MN, et al. BMC Genomics. 2013 Dec 28;14:928. doi: 10.1186/1471-2164-14-928. BMC Genomics. 2013. PMID: 24373541 Free PMC article. - The prominent role of fungi and fungal enzymes in the ant-fungus biomass conversion symbiosis.
Lange L, Grell MN. Lange L, et al. Appl Microbiol Biotechnol. 2014 Jun;98(11):4839-51. doi: 10.1007/s00253-014-5708-5. Epub 2014 Apr 15. Appl Microbiol Biotechnol. 2014. PMID: 24728757 Review. - The origin of the attine ant-fungus mutualism.
Mueller UG, Schultz TR, Currie CR, Adams RM, Malloch D. Mueller UG, et al. Q Rev Biol. 2001 Jun;76(2):169-97. doi: 10.1086/393867. Q Rev Biol. 2001. PMID: 11409051 Review.
Cited by
- Fungiculture in Termites Is Associated with a Mycolytic Gut Bacterial Community.
Hu H, da Costa RR, Pilgaard B, Schiøtt M, Lange L, Poulsen M. Hu H, et al. mSphere. 2019 May 15;4(3):e00165-19. doi: 10.1128/mSphere.00165-19. mSphere. 2019. PMID: 31092601 Free PMC article. - Generation of Nutrients and Detoxification: Possible Roles of Yeasts in Leaf-Cutting Ant Nests.
Mendes TD, Rodrigues A, Dayo-Owoyemi I, Marson FA, Pagnocca FC. Mendes TD, et al. Insects. 2012 Feb 17;3(1):228-45. doi: 10.3390/insects3010228. Insects. 2012. PMID: 26467957 Free PMC article. - The multidimensional nutritional niche of fungus-cultivar provisioning in free-ranging colonies of a neotropical leafcutter ant.
Crumière AJJ, James A, Lannes P, Mallett S, Michelsen A, Rinnan R, Shik JZ. Crumière AJJ, et al. Ecol Lett. 2021 Nov;24(11):2439-2451. doi: 10.1111/ele.13865. Epub 2021 Aug 21. Ecol Lett. 2021. PMID: 34418263 Free PMC article. - Host Susceptibility Modulates Escovopsis Pathogenic Potential in the Fungiculture of Higher Attine Ants.
Jiménez-Gómez I, Barcoto MO, Montoya QV, Goes AC, Monteiro LSVE, Bueno OC, Rodrigues A. Jiménez-Gómez I, et al. Front Microbiol. 2021 Jun 14;12:673444. doi: 10.3389/fmicb.2021.673444. eCollection 2021. Front Microbiol. 2021. PMID: 34194409 Free PMC article. - Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen.
Heine D, Holmes NA, Worsley SF, Santos ACA, Innocent TM, Scherlach K, Patrick EH, Yu DW, Murrell JC, Vieria PC, Boomsma JJ, Hertweck C, Hutchings MI, Wilkinson B. Heine D, et al. Nat Commun. 2018 Jun 7;9(1):2208. doi: 10.1038/s41467-018-04520-1. Nat Commun. 2018. PMID: 29880868 Free PMC article.
References
- Douglas AE. The Symbiotic Habit. Princeton, NJ: Princeton University Press; 2010.
- Agrawal AA, Karban R. Domatia mediate plantarthropod mutualism. Nature. 1997;8:562–563. doi: 10.1038/42384. - DOI
- Nepi M. In: Nectaries and Nectar. Nicolson SW, Nepi M, Pacini E, editor. Dordrecht: Springer; 2007. Nectary structure and ultrastructure; pp. 129–166. full_text.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials