Activation of the maternal immune system induces endocrine changes in the placenta via IL-6 - PubMed (original) (raw)
Activation of the maternal immune system induces endocrine changes in the placenta via IL-6
Elaine Y Hsiao et al. Brain Behav Immun. 2011 May.
Abstract
Activation of the maternal immune system in rodent models sets in motion a cascade of molecular pathways that ultimately result in autism- and schizophrenia-related behaviors in offspring. The finding that interleukin-6 (IL-6) is a crucial mediator of these effects led us to examine the mechanism by which this cytokine influences fetal development in vivo. Here we focus on the placenta as the site of direct interaction between mother and fetus and as a principal modulator of fetal development. We find that maternal immune activation (MIA) with a viral mimic, synthetic double-stranded RNA (poly(I:C)), increases IL-6 mRNA as well as maternally-derived IL-6 protein in the placenta. Placentas from MIA mothers exhibit increases in CD69+ decidual macrophages, granulocytes and uterine NK cells, indicating elevated early immune activation. Maternally-derived IL-6 mediates activation of the JAK/STAT3 pathway specifically in the spongiotrophoblast layer of the placenta, which results in expression of acute phase genes. Importantly, this parallels an IL-6-dependent disruption of the growth hormone-insulin-like growth factor (GH-IGF) axis that is characterized by decreased GH, IGFI and IGFBP3 levels. In addition, we observe an IL-6-dependent induction in pro-lactin-like protein-K (PLP-K) expression as well as MIA-related alterations in other placental endocrine factors. Together, these IL-6-mediated effects of MIA on the placenta represent an indirect mechanism by which MIA can alter fetal development.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Figures
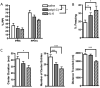
Fig. 1. Maternal IL-6 exposure yields offspring with behavioral abnormalities analogous to those seen in MIA offspring
A. Compared to controls, offspring of mice treated with rIL-6 or poly(I:C) display a PPI deficit [F(2, 74)=4.40, *p < 0.05; n=10 saline, 10 poly(I:C), 19 rIL-6 offspring]. B. rIL-6 and poly(I:C) offspring display increased freezing in response to the conditioned acoustic cue during LI testing, as well as decreased LI compared to saline controls when measured against the non-pre-exposed (NPE) group [% freezing = 64.49 ± 6.05 (mean ± SEM)] [F(2, 41)=3.261, *p < 0.05; n=13 saline, 14 poly(I:C), 17 rIL-6 offspring]. NPE poly(I:C) and saline offspring display no significant difference in freezing response. C. Compared to controls, offspring of rIL-6 or poly(I:C)-injected mothers exhibit fewer entries into the center of the open field [F(2, 92)=8.596; p < 0.0005; n=36 saline, 36 poly(I:C), 23 rIL-6 offspring] and shorter duration spent in the center field [F(2, 92)=3.140; *p < 0.05]. rIL-6 offspring also present shorter total distance traveled in the open field [F(2, 92)=12.81; p < 0.0001].
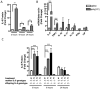
Fig. 2. MIA upregulates maternally-derived IL-6 in the placenta
A. Compared to controls, placentas from poly(I:C)-injected mothers exhibit increased IL-6 protein at 3, 6 and 24 hours post-injection [n= 9 placentas per treatment per time point (pooled from 3 independent litters); * p < 0.05, ** p < 0.001]. B. At 3 hours post-injection, compared to saline controls, placentas from poly(I:C)-injected mothers also display increased mRNA expression of pro-inflammatory cytokines, including a markedly elevated level of IL-6 [n=9 placentas per treatment group (pooled from 3 independent litters); ***p < 0.0001]. C. Eliminating the contribution of fetally-derived IL-6 has no effect on total placental IL-6 levels, whereas eliminating the contribution of maternally-derived IL-6 completely diminishes total placental IL-6 to levels below those seen in saline controls at both 3 hours and 6 hours post-injection. This indicates that basal and MIA-induced placental IL-6 is maternally-derived. [F(3,80)=24.89; ***p < 0.0001; n= 6-15 placentas per treatment group per genotype pair (pooled from 3-6 litters)].
Fig. 3. MIA activates decidual leukocytes to express CD69
A. Compared to controls, placentas from poly(I:C)-injected mothers harbor increased numbers of CD69-expressing CD11b+, Gr-1+ and DBA lectin+ cells [F(1,12)=24.76, ***_p_=0.0003; n=5 litters per treatment group (7-8 deciduas pooled per litter)]. These are similarly increased in poly(I:C)-injected IL-6-/- mothers, suggesting that this effect occurs independently of IL-6 action [F(1,12)=21.87, **_p_=0.0005]. B. Representative flow cytometry spectra show increased total CD69+ cells in poly(I:C) decidual suspensions (left) and increased CD69+ CD11b+ cells from a non-erythroid parent population (right) C. Increased activation of decidual leukocytes corresponds with a trending increase in total CD69+ gene expression (# p = 0.08; n=9 placentas per treatment group (pooled from 3 independent litters)
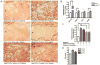
Fig. 4. Maternally-derived IL-6 activates the JAK/STAT3 pathway in the fetal placental compartment in response to MIA
A. Representative sections of the spongiotrophoblast layer of the placenta (with decidua along the top edge and labyrinth along the bottom edge of each image) show positive pSTAT3 staining when placentas are isolated from poly(I:C)-injected mothers. Eliminating the contribution of fetally-derived IL-6 has no effect on pSTAT3 staining, while eliminating the contribution of maternally-derived IL-6 completely abrogates pSTAT3 staining in the spongiotrophoblast. [n=5-7 placentas per treatment group per genotype pair (pooled from 3-5 independent litters)]. Dotted lines = boundary between decidua and spongiotrophoblast layers (upper) and boundary between spongiotrophoblast and labyrinth (lower); D=decidua, Sp=spongiotrophoblast, L=labyrinth; B. MIA-induced JAK/STAT3 activation is accompanied by increased expression of the downstream acute phase genes, SOCS3, TIMP1, Pim1 and NOS2, in poly(I:C) placentas [n=9 placentas per treatment group (pooled from 3 independent litters); ***p < 0.0001, *p < 0.05, # p = 0.07]. C. Eliminating the contribution of maternally-derived IL-6 reduces placental SOCS3 induction [n=9 placentas per treatment group per genotype pair (pooled from 3 independent litters); ***p < 0.0001, *p < 0.05]. D. Murine spongiotrophoblast cells express IL-6Rα and gp130 mRNA, indicating that they may respond directly to placental IL-6 activation. [n=3 saline, 3 poly(I:C} placentas; merged].
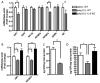
Fig. 5. MIA-induced IL-6 action reduces placental IGFI and IGFBP3
A. Compared to saline controls, placentas from poly(I:C)-injected mothers have significantly reduced expression of IGFI and IGFBP3 mRNA and a trending decrease in GH expression [n=9 placentas per treatment group (pooled from 3 independent litters); **p < 0.001, # p = 0.24]. B. The deficits in IGFI and IGFBP3 expression observed in MIA placentas require IL-6 production [IGFI: F(2,23)=15.95; *p < 0.05; IGFBP3: F(2,23)=37.77; **p < 0.001; n=8-9 placentas per treatment group (pooled from 3 independent litters]. C. MIA placentas exhibit significantly decreased GH protein [n=7 placentas per treatment group (pooled from 3 independent litters); **p < 0.001]. D. MIA placentas exhibit significantly decreased IGFI protein, and this deficiency is dependent on IL-6 production [F(2, 17)=5.908; *p < 0.05; n=5-7 placentas per treatment group (pooled from 3 independent litters)].
Fig. 6. MIA-induced IL-6 action increases PLP-K and related growth factor gene expression
A. Placentas from poly(I:C)-injected mothers have significantly elevated expression of PLP-K and trending increases in PGF and PLII [n=9 per treatment group (pooled from 3 independent litters); *p < 0.05, # p = 0.10, ψ p = 0.11]. B. The upregulation of PLP-K expression observed in MIA placentas require IL-6 action [F(2,23)=5.033; *p < 0.05].
Fig. 7. Model of MIA-induced IL-6 effects on the placenta
Maternal injection of poly(I:C) leads to the activation of the maternal immune system via a TLR3-mediated anti-viral response. Pro-inflammatory factors, including IL-6, are secreted by activated TLR3+ cells into the maternal bloodstream. As maternal blood circulates continuously through the placenta, IL-6 and soluble pro-inflammatory factors increase in the spiral arteries that descend through the decidua and spongiotrophoblast, as well as in the maternal blood spaces of the labyrinth, and circulate back up into the maternal compartment. Resident immune cells in the decidua are activated by maternal cytokines and other signaling factors to express CD69, and they propagate the inflammatory response by further cytokine release. IL-6 derived from decidual cells acts in a paracrine manner on target cells in the spongiotrophoblast layer. Ligation with the cognate receptor IL-6Ra and gp130 leads to signal transduction resulting in JAK/STAT3 activation and downstream changes in gene expression. Increases in acute phase proteins, such as SOCS3, down-regulate placental GH production and signaling. This leads to reduced IGFBP3 and IGFI. Global changes in STAT3 activation in the spongiotrophoblast layer alter the production of placenta-specific PLP and pro-lactin proteins. These changes in endocrine factors lead to acute placental pathophysiology and subsequent effects on fetal development.
Comment in
- How cytokines leave their mark: the role of the placenta in developmental programming of brain and behavior.
Bilbo SD. Bilbo SD. Brain Behav Immun. 2011 May;25(4):602-3. doi: 10.1016/j.bbi.2011.01.018. Epub 2011 Feb 4. Brain Behav Immun. 2011. PMID: 21296146 No abstract available.
Similar articles
- Impact of maternal immune activation and sex on placental and fetal brain cytokine and gene expression profiles in a preclinical model of neurodevelopmental disorders.
Osman HC, Moreno R, Rose D, Rowland ME, Ciernia AV, Ashwood P. Osman HC, et al. J Neuroinflammation. 2024 May 7;21(1):118. doi: 10.1186/s12974-024-03106-7. J Neuroinflammation. 2024. PMID: 38715090 Free PMC article. - Exogenous PD-L1 binds to PD-1 to alleviate and prevent autism-like behaviors in maternal immune activation-induced male offspring mice.
Zeng X, Fan L, Qin Q, Zheng D, Wang H, Li M, Jiang Y, Wang H, Liu H, Liang S, Wu L, Liang S. Zeng X, et al. Brain Behav Immun. 2024 Nov;122:527-546. doi: 10.1016/j.bbi.2024.08.042. Epub 2024 Aug 23. Brain Behav Immun. 2024. PMID: 39182588 - Environmental influences on placental programming and offspring outcomes following maternal immune activation.
Núñez Estevez KJ, Rondón-Ortiz AN, Nguyen JQT, Kentner AC. Núñez Estevez KJ, et al. Brain Behav Immun. 2020 Jan;83:44-55. doi: 10.1016/j.bbi.2019.08.192. Epub 2019 Sep 4. Brain Behav Immun. 2020. PMID: 31493445 Free PMC article. - Molecular mechanisms underlying the models of neurodevelopmental disorders in maternal immune activation relevant to the placenta.
Tsukada T, Shimada H, Sakata-Haga H, Iizuka H, Hatta T. Tsukada T, et al. Congenit Anom (Kyoto). 2019 May;59(3):81-87. doi: 10.1111/cga.12323. Epub 2019 Jan 17. Congenit Anom (Kyoto). 2019. PMID: 30592100 Review. - Innate Immune Responses to Acute Viral Infection During Pregnancy.
Cornish EF, Filipovic I, Åsenius F, Williams DJ, McDonnell T. Cornish EF, et al. Front Immunol. 2020 Sep 30;11:572567. doi: 10.3389/fimmu.2020.572567. eCollection 2020. Front Immunol. 2020. PMID: 33101294 Free PMC article. Review.
Cited by
- Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms.
Love C, Sominsky L, O'Hely M, Berk M, Vuillermin P, Dawson SL. Love C, et al. BMC Med. 2024 Sep 16;22(1):393. doi: 10.1186/s12916-024-03617-3. BMC Med. 2024. PMID: 39278907 Free PMC article. Review. - Does the kynurenine pathway play a pathogenic role in autism spectrum disorder?
Santana-Coelho D. Santana-Coelho D. Brain Behav Immun Health. 2024 Aug 6;40:100839. doi: 10.1016/j.bbih.2024.100839. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39263315 Free PMC article. - Insulin-Like Growth Factor 1 Has the Potential to Be Used as a Diagnostic Tool and Treatment Target for Autism Spectrum Disorders.
Shen J, Liu L, Yang Y, Zhou M, Xu S, Zhang W, Zhang C. Shen J, et al. Cureus. 2024 Jul 25;16(7):e65393. doi: 10.7759/cureus.65393. eCollection 2024 Jul. Cureus. 2024. PMID: 39188438 Free PMC article. Review. - Influenza A virus during pregnancy disrupts maternal intestinal immunity and fetal cortical development in a dose- and time-dependent manner.
Otero AM, Connolly MG, Gonzalez-Ricon RJ, Wang SS, Allen JM, Antonson AM. Otero AM, et al. Mol Psychiatry. 2024 Jul 3. doi: 10.1038/s41380-024-02648-9. Online ahead of print. Mol Psychiatry. 2024. PMID: 38961232 - Gestational hypothyroxinemia induces ASD-like phenotypes in behavior, proinflammatory markers, and glutamatergic protein expression in mouse offspring of both sexes.
González-Madrid E, Rangel-Ramírez MA, Opazo MC, Méndez L, Bohmwald K, Bueno SM, González PA, Kalergis AM, Riedel CA. González-Madrid E, et al. Front Endocrinol (Lausanne). 2024 May 1;15:1381180. doi: 10.3389/fendo.2024.1381180. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38752179 Free PMC article.
References
- Alvir JM, Woerner MG, Gunduz H, Degreef G, Lieberman JA. Obstetric complications predict treatment response in first-episode schizophrenia. Psychol Med. 1999;29:621–627. - PubMed
- Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ. Placental trophoblast inclusions in autism spectrum disorder. Biol Psychiatry. 2007;61:487–491. - PubMed
- Arad M, Atzil S, Shakhar K, Adoni A, Ben-Eliyahu S. Poly I-C induces early embryo loss in f344 rats: a potential role for NK cells. Am J Reprod Immunol. 2005;54:49–53. - PubMed
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behav, Imm. 2010 doi: 10.1016/j.bbi.2010.08.003. in press. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007737-30S1/GM/NIGMS NIH HHS/United States
- F31 MH090749/MH/NIMH NIH HHS/United States
- AS6330/AS/Autism Speaks/United States
- AS1606/AS/Autism Speaks/United States
- 5T32 GM07737/GM/NIGMS NIH HHS/United States
- T32 GM007737/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous