Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells - PubMed (original) (raw)
Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells
Rakesh K Srivastava et al. Front Biosci (Elite Ed). 2011.
Abstract
According to the cancer stem cell hypothesis, the aggressive growth and early metastasis of cancer may arise through dysregulation of self-renewal of stem cells. The objectives of this study were to examine the molecular mechanisms by which sulforaphane (SFN, an active compound in cruciferous vegetables) inhibits self-renewal capacity of pancreatic cancer stem cells (CSCs), and synergizes with quercetin, a major polyphenol and flavonoid commonly detected in many fruits and vegetables. Our data demonstrated that SFN inhibited self-renewal capacity of pancreatic CSCs. Inhibition of Nanog by lentiviral-mediated shRNA expression enhanced the inhibitory effects of sulforaphane on self-renewal capacity of CSCs. SFN induced apoptosis by inhibiting the expression of Bcl-2 and XIAP, phosphorylation of FKHR, and activating caspase-3. Moreover, SFN inhibited expression of proteins involved in the epithelial-mesenchymal transition (beta-catenin, vimentin, twist-1, and ZEB1), suggesting the blockade of signaling involved in early metastasis. Furthermore, the combination of quercetin with SFN had synergistic effects on self-renewal capacity of pancreatic CSCs. These data suggest that SFN either alone or in combination with quercetin can eliminate cancer stem cell-characteristics.
Figures
Figure 1
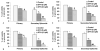
Effects of SFN on spheroid cell viability in cancer stem cells (CSCs) derived from human pancreatic cancer cell lines. (A), Pancreatic CSCs were isolated from MIA PaCa-2 cells, seeded in suspension and treated with SFN (0-10 μM) for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, #, % or ## = significantly different from control, P < 0.05. (B), Pancreatic cancer stem cells were isolated from PANC-1 cells, seeded in suspension and treated with SFN (0-10 μM) for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, #, % or ** = significantly different from control, P < 0.05. (C), Pancreatic cancer stem cells were isolated from AsPC-1 cells. CSCs were seeded in suspension and treated with SFN (0-10 μM) for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, %, # or ## = significantly different from control, P < 0.05. (D), Pancreatic cancer stem cells were isolated from Bx PC-3 cells. CSCs were seeded in suspension and treated with SFN (0-10 μM) for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, #, % or ## = significantly different from control, P < 0.05.
Figure 2
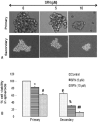
Effects of SFN on tumor spheroids and cell viability of pancreatic cancer stem cells (CSCs). (A), Pancreatic CSCs were seeded in suspension and treated with SFN (0-10 μM) for 7 days. Pictures of spheroids formed in suspension were taken by a microscope. (B), Pancreatic CSCs were seeded in suspension and treated with SFN (0-10 μM) for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, #, % or ## = significantly different from control, P < 0.05.
Figure 3
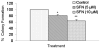
SFN inhibits colony formation by pancreatic CSCs. Pancreatic CSCs were seeded in soft agar and treated with various doses of SFN and incubated at 4°C for 21 days. At the end of incubation period, colonies were counted. Data represent mean ± SD. * or ** = significantly different from respective controls, P < 0.05.
Figure 4
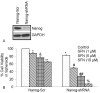
Inhibition of Nanog by shRNA enhances the antiproliferative effects of SFN. Isolated pancreatic CSCs were transduced with either Nanog scrambled or Nanog shRNA. Transduced cells were treated with various doses of SFN and maintained in pancreatic cancer stem cell medium for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, &, #, %, ## or ** = significantly different from control, P < 0.05.
Figure 5
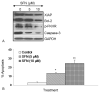
Regulation of apoptosis-related proteins and apoptosis by SFN in pancreatic cancer stem cells. (A), Regulation of apoptosis-related proteins by SFN. Pancreatic CSCs were treated with SFN (0-10 μM) for 48 h. The Western blot analyses were performed to examine the expression of XIAP, Bcl-2, total caspase-3, phospho-FKHR and GAPDH. (B), Regulation of apoptosis by SFN. Pancreatic CSCs were treated with SFN (0-10 μM) for 48 h, and apoptosis was measured by TUNEL assay.
Figure 6
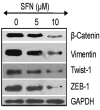
Regulation of epithelial mesenchymal transition factors by SFN in pancreatic cancer stem cells. Pancreatic CSCs were treated with SFN (0-10 μM) for 48 h. At the end of incubation period, the expression of β-catenin, vimentin, Twist-1 and Zeb-1 was measured by the Western blot analysis.
Figure 7
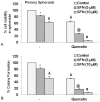
Quercetin synergizes with SFN to inhibit self-renewal capacity of pancreatic cancer CSCs. (A), Quercetin synergizes with SFN to inhibit spheroid cell viability. Pancreatic CSCs were seeded in suspension and treated with SFN (0-10 μM) with or without quercetin (20 μM) for 7 days. At the end of incubation period, all the spheroids were collected and resuspended. Cell viability was measured by trypan blue assay. Data represent mean ± SD. *, &, @ or # * = significantly different from control, P < 0.05. (B), Quercetin synergizes with SFN to inhibit colony formation. SFN inhibits colony formation by pancreatic CSCs. Pancreatic CSCs were seeded in soft agar and treated with various doses of SFN and incubated at 4°C for 21 days. At the end of incubation period, colonies were counted. Data represent mean ± SD. *, &, @ or # = significantly different from respective controls, P < 0.05.
Similar articles
- Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics.
Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Tang SN, et al. Int J Cancer. 2012 Jul 1;131(1):30-40. doi: 10.1002/ijc.26323. Epub 2011 Aug 25. Int J Cancer. 2012. PMID: 21796625 Free PMC article. - Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal.
Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Rodova M, et al. PLoS One. 2012;7(9):e46083. doi: 10.1371/journal.pone.0046083. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029396 Free PMC article. - Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway.
Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Li SH, et al. Mol Cell Biochem. 2013 Jan;373(1-2):217-27. doi: 10.1007/s11010-012-1493-6. Epub 2012 Nov 6. Mol Cell Biochem. 2013. PMID: 23129257 - NANOG: a promising target for digestive malignant tumors.
Sun AX, Liu CJ, Sun ZQ, Wei Z. Sun AX, et al. World J Gastroenterol. 2014 Sep 28;20(36):13071-8. doi: 10.3748/wjg.v20.i36.13071. World J Gastroenterol. 2014. PMID: 25278701 Free PMC article. Review. - The "Big Five" Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein.
Naujokat C, McKee DL. Naujokat C, et al. Curr Med Chem. 2021;28(22):4321-4342. doi: 10.2174/0929867327666200228110738. Curr Med Chem. 2021. PMID: 32107991 Review.
Cited by
- Twist: a molecular target in cancer therapeutics.
Khan MA, Chen HC, Zhang D, Fu J. Khan MA, et al. Tumour Biol. 2013 Oct;34(5):2497-506. doi: 10.1007/s13277-013-1002-x. Epub 2013 Jul 20. Tumour Biol. 2013. PMID: 23873099 Review. - Polyphenols as Possible Agents for Pancreatic Diseases.
Gašić U, Ćirić I, Pejčić T, Radenković D, Djordjević V, Radulović S, Tešić Ž. Gašić U, et al. Antioxidants (Basel). 2020 Jun 23;9(6):547. doi: 10.3390/antiox9060547. Antioxidants (Basel). 2020. PMID: 32585831 Free PMC article. Review. - Pancreatic Cancer and Therapy: Role and Regulation of Cancer Stem Cells.
Barman S, Fatima I, Singh AB, Dhawan P. Barman S, et al. Int J Mol Sci. 2021 Apr 30;22(9):4765. doi: 10.3390/ijms22094765. Int J Mol Sci. 2021. PMID: 33946266 Free PMC article. Review. - Cytotoxic evaluation and chemical investigation of tomatoes from plants (Solanum lycopersicum L.) grown in uncontaminated and experimentally contaminated soils.
Russo C, Barone D, Lavorgna M, Piscitelli C, Macaluso M, Pacifico S, Piccolella S, Giordano A, Isidori M. Russo C, et al. Sci Rep. 2022 Jul 29;12(1):13024. doi: 10.1038/s41598-022-13876-w. Sci Rep. 2022. PMID: 35906264 Free PMC article. - Effective combinations of anti-cancer and targeted drugs for pancreatic cancer treatment.
Nishimoto A. Nishimoto A. World J Gastroenterol. 2022 Jul 28;28(28):3637-3643. doi: 10.3748/wjg.v28.i28.3637. World J Gastroenterol. 2022. PMID: 36161054 Free PMC article. Review.
References
- Warshaw AL, Fernandez-del C. Castillo: Pancreatic carcinoma. N Engl J Med. 1992;326(7):455–65. - PubMed
- Magee CJ, Ghaneh P, Neoptolemos JP. Surgical and medical therapy for pancreatic carcinoma. Best Pract Res Clin Gastroenterol. 2002;16(3):435–55. - PubMed
- Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI, Abrams RA, Laheru D, Jaffee EM, Hidalgo M, Yeo CJ. Pancreatic cancer. Curr Probl Cancer. 2002;26(4):176–275. - PubMed
- Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96(8):583–5. - PubMed
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA114469/CA/NCI NIH HHS/United States
- R01CA114469/CA/NCI NIH HHS/United States
- R01CA125262/CA/NCI NIH HHS/United States
- R01CA125262-02S1/CA/NCI NIH HHS/United States
- R01 CA125262/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous