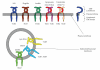Current views of toll-like receptor signaling pathways - PubMed (original) (raw)
Current views of toll-like receptor signaling pathways
Masahiro Yamamoto et al. Gastroenterol Res Pract. 2010.
Abstract
On microbial invasion, the host immediately evokes innate immune responses. Recent studies have demonstrated that Toll-like receptors (TLRs) play crucial roles in innate responses that lead not only to the clearance of pathogens but also to the efficient establishment of acquired immunity by directly detecting molecules from microbes. In terms of intracellular TLR-mediated signaling pathways, cytoplasmic adaptor molecules containing Toll/IL-1R (TIR) domains play important roles in inflammatory immune responses through the production of proinflammatory cytokines, nitric oxide, and type I interferon, and upregulation of costimulatory molecules. In this paper, we will describe our current understanding of the relationship between TLRs and their ligands derived from pathogens such as viruses, bacteria, fungi, and parasites. Moreover, we will review the historical and current literature to describe the mechanisms behind TLR-mediated activation of innate immune responses.
Figures
Figure 1
Extracellular and intracellular TLRs. TLRs can be divided into extracellular and intracellular TLRs. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11 recognize their ligands on the cell surface. On the other hand, TLR3, TLR7, TLR8, and TLR9 are intracellularly localized.
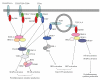
Figure 2
TLR-mediated MyD88-dependent or TRIF-dependent pathways. TIR domain-containing adaptors MyD88, TIRAP, TRIF, and TRAM define TLR-mediated signaling. MyD88 and TIRAP are adaptors for the MyD88-dependent pathways, which mainly activate proinflammatory cytokine production. On the other hand, TRIF and TRAM are adaptors for IRF3 activation, resulting in production of type I IFN by TLR3- or TLR4-mediated TRIF-dependent pathways. In pDCs, TLR7/TLR9-mediated MyD88-dependent pathways induce IRF7 activation, leading to IFN_α_ production.
Similar articles
- Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review. - Microbial recognition by Toll-like receptors.
Takeda K, Akira S. Takeda K, et al. J Dermatol Sci. 2004 Apr;34(2):73-82. doi: 10.1016/j.jdermsci.2003.10.002. J Dermatol Sci. 2004. PMID: 15033189 Review. - [Frontier of mycobacterium research--host vs. mycobacterium].
Okada M, Shirakawa T. Okada M, et al. Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese. - Toll-like receptors as adjuvant receptors.
Kaisho T, Akira S. Kaisho T, et al. Biochim Biophys Acta. 2002 Feb 13;1589(1):1-13. doi: 10.1016/s0167-4889(01)00182-3. Biochim Biophys Acta. 2002. PMID: 11909637 Review. - Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM.
Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, Taylor MJ, Golenbock DT, Fitzgerald KA, Kazura JW, Pearlman E. Hise AG, et al. J Immunol. 2007 Jan 15;178(2):1068-76. doi: 10.4049/jimmunol.178.2.1068. J Immunol. 2007. PMID: 17202370
Cited by
- Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease.
Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Machado FS, et al. Semin Immunopathol. 2012 Nov;34(6):753-70. doi: 10.1007/s00281-012-0351-7. Epub 2012 Oct 18. Semin Immunopathol. 2012. PMID: 23076807 Free PMC article. Review. - Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms.
Yang L, Seki E. Yang L, et al. Front Physiol. 2012 May 22;3:138. doi: 10.3389/fphys.2012.00138. eCollection 2012. Front Physiol. 2012. PMID: 22661952 Free PMC article. - Host-microbe interaction: Innate immunity cues virulence.
Buckner MM, Finlay BB. Buckner MM, et al. Nature. 2011 Apr 14;472(7342):179-80. doi: 10.1038/472179a. Nature. 2011. PMID: 21490667 No abstract available. - M1 and M2 immune activation in Parkinson's Disease: Foe and ally?
Moehle MS, West AB. Moehle MS, et al. Neuroscience. 2015 Aug 27;302:59-73. doi: 10.1016/j.neuroscience.2014.11.018. Epub 2014 Nov 25. Neuroscience. 2015. PMID: 25463515 Free PMC article. Review. - Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis.
Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, Thalheimer U. Giannelli V, et al. World J Gastroenterol. 2014 Dec 7;20(45):16795-810. doi: 10.3748/wjg.v20.i45.16795. World J Gastroenterol. 2014. PMID: 25492994 Free PMC article. Review.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. - PubMed
- Beutler B, Eidenschenk C, Crozat K, et al. Genetic analysis of resistance to viral infection. Nature Reviews Immunology. 2007;7(10):753–766. - PubMed
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. - PubMed
- Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symposia on Quantitative Biology. 1989;54(1):1–13. - PubMed
- Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources