Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease - PubMed (original) (raw)
Review
Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease
Nels Olson et al. Nitric Oxide. 2011.
Abstract
Induction and activation of nitric oxide (NO) synthases (NOS) and excessive production of NO are common features of almost all diseases associated with infection and acute or chronic inflammation, although the contribution of NO to the pathophysiology of these diseases is highly multifactorial and often still a matter of controversy. Because of its direct impact on tissue oxygenation and cellular oxygen (O(2)) consumption and re-distribution, the ability of NO to regulate various aspects of hypoxia-induced signaling has received widespread attention. Conditions of tissue hypoxia and the activation of hypoxia-inducible factors (HIF) have been implicated in hypoxia or in cancer biology, but are also being increasingly recognized as important features of acute and chronic inflammation. Thus, the activation of HIF transcription factors has been increasingly implicated in inflammatory diseases, and recent studies have indicated its critical importance in regulating phagocyte function, inflammatory mediator production, and regulation of epithelial integrity and repair processes. Finally, HIF also appears to contribute to important features of tissue fibrosis and epithelial-to-mesenchymal transition, processes that are associated with tissue remodeling in various non-malignant chronic inflammatory disorders. In this review, we briefly summarize the current state of knowledge with respect to the general mechanisms involved in HIF regulation and the impact of NO on HIF activation. Secondly, we will summarize the major recent findings demonstrating a role for HIF signaling in infection, inflammation, and tissue repair and remodeling, and will address the involvement of NO. The growing interest in hypoxia-induced signaling and its relation with NO biology is expected to lead to further insights into the complex roles of NO in acute or chronic inflammatory diseases and may point to the importance of HIF signaling as key feature of NO-mediated events during these disorders.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
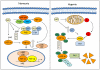
Figure 1. Schematic representation of the mechanisms by which NO impacts on HIF activation during normoxic or hypoxic conditions
Under normoxia (left panel) NO can inhibit prolyl hydroxylase (PHD) and factor inhibiting HIF (FIH) activity by interacting with enzyme bound Fe2+, preventing hydroxylation of HIF-1α proline and asparagine residues that target HIF-1α for degradation and block interaction with the transcriptional co-activator p300-CBP. NO can also increase HIF-1α expression through the activation of PI3K/AKT and MAPK pathways and stabilize HIF-1α by _S_-nitrosylation. _S_-nitrosylation of von Hippel Lindau (VHL) can also prevent HIF-1α ubiquitination and degradation. During hypoxic conditions (right panel), competitive binding of NO to mitochondrial cytochrome c oxidase results in re-distribution of O2 to restore PHD activity. NO can also promote increases in intracellular free iron that enhance PHD activity. p300-CBP, p300-CREB-binding protein; FIH, factor inhibiting HIF; HIF-1α, hypoxia inducible factor-1α; HRE; hypoxic response element, PHD, prolyl hydroxylase; PI3K, phosphatidylinositol 3-kinase; VHL, von Hippel Lindau.
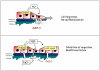
Figure 2. Schematic model of proposed actions of NO in airway epithelial regeneration following injury, and the involvement of HIF
Initial stages of epithelial repair may be promoted by low concentrations of NO from NOS2, which promote epithelial cell migration in part by induction of repair genes such as matrix metalloproteinase-9 (top panel). At later stages, elevated concentrations of NO may attenuate cell migration by suppressing MMP-9 expression and activation (through induction of PAI-1) and by activating p53 (lower panel). As indicated, some of these responses are mediated by the stabilization and activation of HIF-1 [87]. This biphasic control of cell migration by NO may be critical for a coordinated wound response and appropriate re-epithelialization. HIF-1α hypoxia inducible factor-1α; MMP-9 matrix metalloproteinase 9; NOS2, nitric oxide synthase2, PAI-1; plasminogen activator inhibitor-1.
Similar articles
- Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms.
Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Korbecki J, et al. Int J Mol Sci. 2021 Oct 2;22(19):10701. doi: 10.3390/ijms221910701. Int J Mol Sci. 2021. PMID: 34639040 Free PMC article. Review. - Modulation of NF-κB and hypoxia-inducible factor--1 by S-nitrosoglutathione does not alter allergic airway inflammation in mice.
Olson N, Kasahara DI, Hristova M, Bernstein R, Janssen-Heininger Y, van der Vliet A. Olson N, et al. Am J Respir Cell Mol Biol. 2011 Jun;44(6):813-23. doi: 10.1165/rcmb.2010-0035OC. Epub 2010 Aug 6. Am J Respir Cell Mol Biol. 2011. PMID: 20693401 Free PMC article. - Nitric oxide modulates hypoxia-inducible factor-1 and poly(ADP-ribose) polymerase-1 cross talk in response to hypobaric hypoxia.
Martínez-Romero R, Cañuelo A, Siles E, Oliver FJ, Martínez-Lara E. Martínez-Romero R, et al. J Appl Physiol (1985). 2012 Mar;112(5):816-23. doi: 10.1152/japplphysiol.00898.2011. Epub 2011 Dec 15. J Appl Physiol (1985). 2012. PMID: 22174393 - HIF-1 in the inflammatory microenvironment.
Dehne N, Brüne B. Dehne N, et al. Exp Cell Res. 2009 Jul 1;315(11):1791-7. doi: 10.1016/j.yexcr.2009.03.019. Epub 2009 Mar 28. Exp Cell Res. 2009. PMID: 19332053 Review. - Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor-1 alpha.
Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Bove PF, et al. J Biol Chem. 2008 Jun 27;283(26):17919-28. doi: 10.1074/jbc.M709914200. Epub 2008 Apr 18. J Biol Chem. 2008. PMID: 18424783 Free PMC article.
Cited by
- The Smell of Hypoxia: using an electronic nose at altitude and proof of concept of its role in the prediction and diagnosis of acute mountain sickness.
Lacey JRN, Kidel C, van der Kaaij JM, Brinkman P, Gilbert-Kawai ET, Grocott MPW, Mythen MG, Martin DS; Xtreme Everest 2 Research Group. Lacey JRN, et al. Physiol Rep. 2018 Sep;6(17):e13854. doi: 10.14814/phy2.13854. Physiol Rep. 2018. PMID: 30187693 Free PMC article. - Evaluation of the Nano-TiO2 as a Novel Deswelling Material.
Chu M, Hou YL, Xu L, Chu ZY, Zhang MB, Wang YD. Chu M, et al. Molecules. 2016 Jan 4;21(1):57. doi: 10.3390/molecules21010057. Molecules. 2016. PMID: 26742025 Free PMC article. - Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties.
Reddy VD, Padmavathi P, Kavitha G, Saradamma B, Varadacharyulu N. Reddy VD, et al. Mol Cell Biochem. 2013 Mar;375(1-2):39-47. doi: 10.1007/s11010-012-1526-1. Epub 2012 Dec 1. Mol Cell Biochem. 2013. PMID: 23212448 - Antitumor and antiviral activities of 4-substituted 1,2,3-triazolyl-2,3-dibenzyl-L-ascorbic acid derivatives.
Macan AM, Harej A, Cazin I, Klobučar M, Stepanić V, Pavelić K, Pavelić SK, Schols D, Snoeck R, Andrei G, Raić-Malić S. Macan AM, et al. Eur J Med Chem. 2019 Dec 15;184:111739. doi: 10.1016/j.ejmech.2019.111739. Epub 2019 Sep 28. Eur J Med Chem. 2019. PMID: 31586832 Free PMC article. - Anthrax lethal toxin inhibits translation of hypoxia-inducible factor 1α and causes decreased tolerance to hypoxic stress.
Ouyang W, Torigoe C, Fang H, Xie T, Frucht DM. Ouyang W, et al. J Biol Chem. 2014 Feb 14;289(7):4180-90. doi: 10.1074/jbc.M113.530006. Epub 2013 Dec 23. J Biol Chem. 2014. PMID: 24366872 Free PMC article.
References
- Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adnosin receptors. Nat Rev Immunol. 2005;5:712–21. - PubMed
- Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. - PubMed
- Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 ES007122-25/ES/NIEHS NIH HHS/United States
- T32 ES007122/ES/NIEHS NIH HHS/United States
- HL085646/HL/NHLBI NIH HHS/United States
- HL074295/HL/NHLBI NIH HHS/United States
- R01 HL074295/HL/NHLBI NIH HHS/United States
- R01 HL068865/HL/NHLBI NIH HHS/United States
- R01 HL068865-05/HL/NHLBI NIH HHS/United States
- R01 HL085646/HL/NHLBI NIH HHS/United States
- R01 HL085646-03/HL/NHLBI NIH HHS/United States
- HL068865/HL/NHLBI NIH HHS/United States
- T32 ES007122-30/ES/NIEHS NIH HHS/United States
- R01 HL074295-04/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources