Sugar-free frosting, a homolog of SAD kinase, drives neural-specific glycan expression in the Drosophila embryo - PubMed (original) (raw)
Sugar-free frosting, a homolog of SAD kinase, drives neural-specific glycan expression in the Drosophila embryo
Sarah Baas et al. Development. 2011 Feb.
Abstract
Precise glycan structures on specific glycoproteins impart functionalities essential for neural development. However, mechanisms controlling embryonic neural-specific glycosylation are unknown. A genetic screen for relevant mutations in Drosophila generated the sugar-free frosting (sff) mutant that reveals a new function for protein kinases in regulating substrate flux through specific Golgi processing pathways. Sff is the Drosophila homolog of SAD kinase, which regulates synaptic vesicle tethering and neuronal polarity in nematodes and vertebrates. Our Drosophila sff mutant phenotype has features in common with SAD kinase mutant phenotypes in these other organisms, but we detect altered neural glycosylation well before the initiation of embryonic synaptogenesis. Characterization of Golgi compartmentation markers indicates altered colocalization that is consistent with the detected shift in glycan complexity in sff mutant embryos. Therefore, in analogy to synaptic vesicle tethering, we propose that Sff regulates vesicle tethering at Golgi membranes in the developing Drosophila embryo. Furthermore, neuronal sff expression is dependent on transcellular signaling through a non-neural toll-like receptor, linking neural-specific glycan expression to a kinase activity that is induced in response to environmental cues.
Figures
Fig. 1.
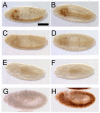
HRP-epitope expression is decreased in _sff_B22 embryos. (A) HRP epitopes of the Drosophila embryo are N-linked glycans that share an antigenic determinant: Fuc in α3-linkage to the innermost GlcNAc of the chitobiose core. Graphical representations of monosaccharides and glycan structures in all figures are in accordance with the recommendations of the Consortium for Functional Glycomics. (B-D) Anti-HRP staining of wild-type Drosophila embryos is shown at progressively later stages, anterior to the left. HRP epitope is visible along the ventral nerve cord (vnc), the hindgut (hg) and the garland gland (gg). Peripheral nervous system (pns) staining is first apparent at Stage 14, but clearly detectable at stage 16. (E-G) In _sff_B22 embryos at 25°C, staining is only detected at the dorsal surface of the nerve cord, along the axon scaffold and in the garland gland. Hindgut staining is not detected. (H-J) At 21°C, the appearance of residual HRP epitope in the sff mutant is delayed in comparison with that observed in mutants at 25°C. Scale bar: 60 μm for B-J.
Fig. 2.
The sff mutation is uncovered by the Δ_brm_11 deficiency and _sff_B22 is an hypomorphic allele that is rescued by transgenic expression of CG6114. (A-F) A late stage 14 _sff_B22 embryo (anterior to the left) stained with anti-HRP antibody exhibits epitope distribution characteristic for the mutant (A,B). A late stage 14 embryo of the genotype _sff_B22/Δ_brm_11 (C,D) shows reduced staining compared with the _sff_B22 homozygote (A,B). A late stage embryo homozygous for the Δ_brm_11 deletion shows complete loss of HRP epitope (E,F). Embryos in A-D were processed in parallel. A, C and E show lateral views; B, D and F show ventral views. (G,H) Lateral views of stage 12 embryos of genotype elav-Gal4; +/+; _sff_B22/_sff_B22 (G) and elav-Gal4; UAS-CG6114/+; _sff_B22/_sff_B22 (H), demonstrating rescue of HRP-epitope expression by transgenic Sff. Scale bar: 120 μm for all panels.
Fig. 3.
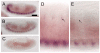
Drosophila sff mRNA is expressed in the embryonic nervous system and is reduced in _sff_B22. (A-E) In situ hybridization (ISH) with anti-sense probe for CG6114 (sff) to late stage 13 wild-type embryo (A,D) reveals prominent message expression in the ventral nerve cord and in sensory neurons in the PNS (arrows in D). ISH on a late stage 13 _sff_B22 embryo shows decreased mRNA levels in the ventral nerve cord (B) and PNS (E). Arrow indicates low staining in sensory neurons of the PNS. No hybridization was observed with the sense probe (C; late stage 13 wild-type embryo). Anterior is to the left. Embryos presented in all panels were processed in parallel. Scale bar: 60 μm for A-C; 15 μm for D,E.
Fig. 4.
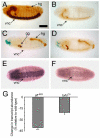
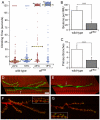
sff interacts with Tollo and expression of sff mRNA is dependent on Tollo. (A) Wild-type embryos and embryos heterozygous for either Tollo or _sff_B22 are identical in appearance, with strong staining of the ventral nerve cord (vnc), garland gland (gg) and hindgut (hg). (B) Embryos homozygous for the _sff_B22 mutation exhibit reduced vnc and hg staining. (C) Embryos homozygous for loss of Tollo lack vnc staining but retain gg and hg expression of the HRP epitope. (D) Embryos that are transheterozygous for both _sff_B22 and Tollo have a composite staining phenotype, indicating functional interactions between the two genes. (E,F) In situ hybridization of antisense probe for sff in wild-type (E) and Tollo mutants (F) indicates that Tollo signaling induces neuronal expression of sff mRNA. Scale bar: 90 μm for A-F. (G) qRT-PCR of sff transcript levels in sff (_sff_B22) and Tollo (_tollo_c5) mutant embryos, relative to wild type *P<0.05, ***P<0.01; mean ± s.e.m. for three independent analyses.
Fig. 5.
Sff mutants exhibit behavioral and neuromuscular junction (NMJ) phenotypes. (A) Drosophila Sff mutants are deficient in negative geotaxis. Each dot represents the climbing time for a single adult male to reach a pre-specified height. Dashed lines indicate the median climbing time for the indicated population. Individuals that did not reach the threshold height within 120 seconds are binned together (*). For all populations, _n_=100-122 males. (B,C) The number of boutons and the number of primary nerve branches are significantly decreased at the NMJ in the _sff_B22 mutant. Data are mean ± s.e.m. ***P<0.001; _n_=25 wild-type, _n_=7 _sff_B22 for panel B and _n_=23 wild-type, _n_=22 _sff_B22 for panel C. (D,E) Compressed _z_-stacks from confocal images of the larval NMJ in wild type (D) and _sff_B22 (E) present the gross change in morphology resulting from decreased Sff function (red, phalloidin; green, anti-HRP). In addition to decreased bouton number and decreased nerve terminal branching, the _sff_B22 NMJ is characterized by enlarged boutons (arrows in D and E). (F,G) The distribution of active zone complexes is not altered by the sff mutation. Wild-type (F) and _sff_B22 (G) larval NMJs were stained with anti-Bruchpilot (green) and anti-HRP (red). Neither the size of Bruchpilot complexes nor the number of complexes per bouton was significantly altered (insets). In panels D-G anti-HRP staining delineates the full extent of the axonal arborization. Despite reduced HRP epitope in embryos and larvae, anti-HRP antibody staining is co-extensive with mAb 1D4 staining, another well-established NMJ marker (see Fig. S8 in the supplementary material). Scale bar: 20 μm for D,E; 15 μm for F,G; 4 μm for insets in F,G.
Fig. 6.
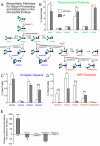
The N-linked glycan profile of the _sff_B22 mutant is deficient in HRP epitopes and shifted towards greater complexity. (A) N-linked glycan processing in Drosophila proceeds through steps that are well conserved across animal species. See Fig. 1 for key to graphical representation of structures. GlcNAcTransferase-1 (GlcNAcT1) transfers a GlcNAc residue to the trimmed high-mannose structure M5N2. Subsequent removal of Man residues by Golgi mannosidases produces NM3N2, a structural node that feeds into three separate pathways. The Fused Lobes hexosaminidase (Fdl) can remove the GlcNAc added by GlcNAcT1 to generate the paucimannose glycan M3N2. Alternatively, NM3N2 can be branched by the action of another GlcNAcTransferase (GlcNAcT2 or GlcNAcT4) to produce complex glycans that are resistant to degradation by Fdl. The NM3N2 glycan is also a substrate for α6-fucosylation (FucT6) and subsequent branching by GlcNAcT2 or GlcNAcT4. Addition of α3-Fuc residues (FucTA) generates HRP epitopes. (B-D) The prevalences of the indicated glycans are given as the percent that they contribute to the total profile of N-linked glycans (% Total Profile, see Table S4 in the supplementary material for full glycan profiles). (E) qRT-PCR of glycogene transcript levels in sff mutant embryos relative to wild type *P<0.05, **P<0.02, ***P<0.01. Values are the mean ± s.e.m. for three independent analyses in panels B-E.
Fig. 7.
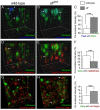
Neuronal Golgi compartmentation is altered in _sff_B22. (A-F) Representative three-dimensional reconstructions are shown of portions of regions of interest that were used to quantify Golgi marker colocalization in the ventral nerve cord of wild-type (A,D,G) and _sff_B22 (B,E,H) Drosophila embryos. Preparations were triple stained with PNA lectin (a trans Golgi marker; green), mAb 1D4 (Fas2, an HRP protein; blue) and anti-YFP (a medial/trans Golgi marker; red). Objects labeled for Fas2 and the trans marker are closely apposed (arrows in A) but exhibit less overlap in the wild-type nerve cord (arrowheads in A) than in the _sff_B22 mutant (B). Almost all the Fas2 signal overlaps with the trans marker in the mutant (arrows in B). Fluorescence intensity colocalization was quantified (C) within regions of interest for wild-type and _sff_B22 mutant embryos (mean ± s.e.m. for _n_=24 segments for each genotype). Fas2 signal is tightly associated with the trans marker in _sff_B22 [mean Pearson's correlation coefficient (PCC)=0.62±0.14], demonstrating a statistically significant increase over the colocalization of these markers in wild type (PCC=0.45±0.12). Consistent with increased polarization between Golgi compartments, trans and medial/trans markers display less overlap in _sff_B22 mutant embryos (arrows in D and E, quantified in F, PCC=0.19±0.03 and 0.31±0.05 in _sff_B22 and wild type, respectively, _n_=24 segments for each genotype). (G-I) Preparations were double stained (G,H) with PNA lectin (green) and anti-GM130 (a Golgin localized to the cis Golgi; red). Unlike the medial/trans marker, the cis marker GM130 displays increased overlap with the trans compartment in _sff_B22 mutant embryos (arrows in G and H, quantified in I; PCC=0.46±0.14 and 0.05±0.03 in _sff_B22 and wild type, respectively, _n_=12 segments for wild-type and _n_=7 segments for _sff_B22). Regions of interest were selected to eliminate any contribution of axon staining to the final quantification (see Figs S9, S10 in the supplementary material). Gridlines correspond to 1 μm in all reconstructions. ***P<0.002.
Fig. 8.
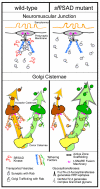
Parallels between known functions for Sff/SAD at the presynaptic membrane of the neuromuscular junction (NMJ) and proposed functions for Sff/SAD at cisternal membranes of the Golgi apparatus. At the NMJ, SAD kinase facilitates the tethering of synaptic vesicles at presynaptic active zones. The tethering complex brings together molecular components necessary for priming and fusion of synaptic vesicles. Presynaptic tethering complexes comprise spatially extended tethering factors such as Bruchpilot (a Drosophila protein that combines the signature domains of the vertebrate Bassoon/Piccolo and ERKs/CAST family members) and other scaffold or tethering components. In the absence of SAD kinase, synaptic vesicles are inefficiently associated with the active zone. Altered glycan expression in the Drosophila sff mutant indicates that Sff/SAD kinase serves an analogous function at Golgi cisternal membranes, ensuring that specific Golgi transport vesicles associate with appropriate stack-specific tethering factors such as Golgin family members. In the sff mutant, Golgi vesicular transport is not halted because secondary cisternal targets are available and competent for fusion. But, the resulting aberrant trafficking shifts glycan profiles by allowing access of glycoprotein substrates to alternative ensembles of processing enzymes (Golgi glycosyltransferases). In _sff_B22, decreased activity reduces HRP-epitope expression (orange processing pathway) and increases complex glycan production (green processing pathway).
Similar articles
- COG7 deficiency in Drosophila generates multifaceted developmental, behavioral and protein glycosylation phenotypes.
Frappaolo A, Sechi S, Kumagai T, Robinson S, Fraschini R, Karimpour-Ghahnavieh A, Belloni G, Piergentili R, Tiemeyer KH, Tiemeyer M, Giansanti MG. Frappaolo A, et al. J Cell Sci. 2017 Nov 1;130(21):3637-3649. doi: 10.1242/jcs.209049. Epub 2017 Sep 7. J Cell Sci. 2017. PMID: 28883096 Free PMC article. - Distinct functional units of the Golgi complex in Drosophila cells.
Yano H, Yamamoto-Hino M, Abe M, Kuwahara R, Haraguchi S, Kusaka I, Awano W, Kinoshita-Toyoda A, Toyoda H, Goto S. Yano H, et al. Proc Natl Acad Sci U S A. 2005 Sep 20;102(38):13467-72. doi: 10.1073/pnas.0506681102. Epub 2005 Sep 8. Proc Natl Acad Sci U S A. 2005. PMID: 16174741 Free PMC article. - Synaptic roles for phosphomannomutase type 2 in a new Drosophila congenital disorder of glycosylation disease model.
Parkinson WM, Dookwah M, Dear ML, Gatto CL, Aoki K, Tiemeyer M, Broadie K. Parkinson WM, et al. Dis Model Mech. 2016 May 1;9(5):513-27. doi: 10.1242/dmm.022939. Epub 2016 Mar 3. Dis Model Mech. 2016. PMID: 26940433 Free PMC article. - Functional analysis of glycosylation using Drosophila melanogaster.
Nishihara S. Nishihara S. Glycoconj J. 2020 Feb;37(1):1-14. doi: 10.1007/s10719-019-09892-0. Epub 2019 Nov 26. Glycoconj J. 2020. PMID: 31773367 Review. - The role of protein N-glycosylation in neural transmission.
Scott H, Panin VM. Scott H, et al. Glycobiology. 2014 May;24(5):407-17. doi: 10.1093/glycob/cwu015. Epub 2014 Mar 18. Glycobiology. 2014. PMID: 24643084 Free PMC article. Review.
Cited by
- Identification and Characterization of Breakpoints and Mutations on Drosophila melanogaster Balancer Chromosomes.
Miller DE, Kahsai L, Buddika K, Dixon MJ, Kim BY, Calvi BR, Sokol NS, Hawley RS, Cook KR. Miller DE, et al. G3 (Bethesda). 2020 Nov 5;10(11):4271-4285. doi: 10.1534/g3.120.401559. G3 (Bethesda). 2020. PMID: 32972999 Free PMC article. - Toll-8/Tollo negatively regulates antimicrobial response in the Drosophila respiratory epithelium.
Akhouayri I, Turc C, Royet J, Charroux B. Akhouayri I, et al. PLoS Pathog. 2011 Oct;7(10):e1002319. doi: 10.1371/journal.ppat.1002319. Epub 2011 Oct 13. PLoS Pathog. 2011. PMID: 22022271 Free PMC article. - Genome-Wide Association of Heroin Dependence in Han Chinese.
Kalsi G, Euesden J, Coleman JR, Ducci F, Aliev F, Newhouse SJ, Liu X, Ma X, Wang Y, Collier DA, Asherson P, Li T, Breen G. Kalsi G, et al. PLoS One. 2016 Dec 9;11(12):e0167388. doi: 10.1371/journal.pone.0167388. eCollection 2016. PLoS One. 2016. PMID: 27936112 Free PMC article. - COG7 deficiency in Drosophila generates multifaceted developmental, behavioral and protein glycosylation phenotypes.
Frappaolo A, Sechi S, Kumagai T, Robinson S, Fraschini R, Karimpour-Ghahnavieh A, Belloni G, Piergentili R, Tiemeyer KH, Tiemeyer M, Giansanti MG. Frappaolo A, et al. J Cell Sci. 2017 Nov 1;130(21):3637-3649. doi: 10.1242/jcs.209049. Epub 2017 Sep 7. J Cell Sci. 2017. PMID: 28883096 Free PMC article. - Changes in Presynaptic Gene Expression during Homeostatic Compensation at a Central Synapse.
Harrell ER, Pimentel D, Miesenböck G. Harrell ER, et al. J Neurosci. 2021 Apr 7;41(14):3054-3067. doi: 10.1523/JNEUROSCI.2979-20.2021. Epub 2021 Feb 19. J Neurosci. 2021. PMID: 33608385 Free PMC article.
References
- Allendoerfer K. L., Durairaj A., Matthews G. A., Patterson P. H. (1999). Morphological domains of Lewis-X/FORSE-1 immunolabeling in the embryonic neural tube are due to developmental regulation of cell surface carbohydrate expression. Dev. Biol. 211, 208-219 - PubMed
- Aoki K., Perlman M., Lim J., Cantu R., Wells L., Tiemeyer M. (2007). Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 282, 9127-9142 - PubMed
- Barnes A. P., Lilley B. N., Pan Y. A., Plummer L. J., Powell A. W., Raines A. N., Sanes J. R., Polleux F. (2007). LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129, 549-563 - PubMed
- Bieberich E., Freischutz B., Liour S. S., Yu R. K. (1998). Regulation of ganglioside metabolism by phosphorylation and dephosphorylation. J. Neurochem. 71, 972-979 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases