Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling - PubMed (original) (raw)
Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling
Yun-Feng Li et al. J Neurosci. 2011.
Abstract
Phosphodiesterase-4 (PDE4) plays an important role in mediating memory via the control of intracellular cAMP signaling; inhibition of PDE4 enhances memory. However, development of PDE4 inhibitors as memory enhancers has been hampered by their major side effect of emesis. PDE4 has four subtypes (PDE4A-D) consisting of 25 splice variants. Mice deficient in PDE4D displayed memory enhancement in radial arm maze, water maze, and object recognition tests. These effects were mimicked by repeated treatment with rolipram in wild-type mice. In addition, similarly as rolipram-treated wild-type mice, PDE4D-deficient mice also displayed increased hippocampal neurogenesis and phosphorylated cAMP response element-binding protein (pCREB). Furthermore, microinfusion of lentiviral vectors that contained microRNAs (miRNAs) targeting long-form PDE4D isoforms into bilateral dentate gyri of the mouse hippocampus downregulated PDE4D4 and PDE4D5, enhanced memory, and increased hippocampal neurogenesis and pCREB. Finally, while rolipram and PDE4D deficiency shortened α2 adrenergic receptor-mediated anesthesia, a surrogate measure of emesis, miRNA-mediated PDE4D knock-down in the hippocampus did not. The present results suggest that PDE4D, in particular long-form PDE4D, plays a critical role in the mediation of memory and hippocampal neurogenesis, which are mediated by cAMP/CREB signaling; reduced expression of PDE4D, or at least PDE4D4 and PDE4D5, in the hippocampus enhances memory but appears not to cause emesis. These novel findings will aid in the development of PDE4 subtype- or variant-selective inhibitors for treatment of disorders involving impaired cognition, including Alzheimer's disease.
Figures
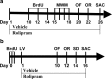
Figure 1.
Schedules of drug treatments and tests. a, Treatment schedule and test order for mice deficient in PDE4D and their wild-type controls. BrdU (100 mg/kg) was injected (i.p.) once daily on days 10, 12, and 14 after rolipram treatment. Behavioral tests were carried out on days 15–22 (1 h after the injection of rolipram or vehicle). b, Treatment schedule and test order for mice treated with lentiviral vectors. BrdU was injected three times with 3 h intervals. The next day, lentiviral vectors containing 4DmiRs or the NC sequence were infused into bilateral dentate gyri (4 × 106 TU/μl/side) of the mouse hippocampus. Behavioral tests were carried out on days 10–14. Rolipram (1.25 mg/kg) or its vehicle (saline containing 5% DMSO) was injected (i.p.) once daily for 26 d (a) or 16 d (b), after which the animals were killed. MWM, Morris water maze; OF, open field; OR, object recognition; LV, lentiviral vectors; SD, step-down passive avoidance; SAC, sacrifice.
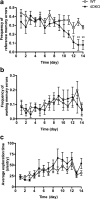
Figure 2.
a–c, Mice deficient in PDE4D (4DKO) displayed decreases in frequency of reference memory errors (a) but not frequency of working memory errors (b) or average exploration time (c) in the eight-arm radial maze task. The frequency of reference or working memory errors was calculated as reference memory errors and working memory errors, respectively, divided by the total number of arm entries; the average exploration time was calculated as the test duration divided by the total number of arm entries. Mice were trained for two sessions a day for 14 successive days. Values shown are means ± SEM; n = 6–7. **p < 0.01 versus the WT control.
Figure 3.
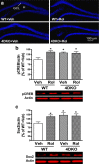
Memory enhancement in mice deficient in PDE4D (4DKO) with or without rolipram (Rol) treatment. a, Escape latency during the acquisition trials (6 trials × 2 d plus 4 trials × 1 d) in the water maze test in 4DKO mice and their WT littermates. No difference between 4DKO and WT or rolipram and vehicle (Veh) in the same genotype. b, Increased entries and duration in the target quadrant in the probe trial of the water maze test in 4DKO mice or WT mice treated with rolipram. c, Increased recognition index in the object recognition test in 4DKO mice or WT mice treated with rolipram. The recognition index was the exploration time in the novel object, _T_n, divided by the total exploration time (i.e., _T_n plus the exploration time in the familiar object, _T_f). Rolipram (1.25 mg/kg) or vehicle was injected (i.p.) once daily for 15–18 d (a, b) or 22 d (c) before the test. Values shown are means ± SEM; n = 7–8; *p < 0.05, ** p < 0.01 versus corresponding WT plus vehicle.
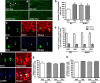
Figure 4.
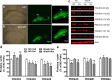
Cells labeled for BrdU and/or NeuN, S100β, PSA-NCAM, or pCREB in the hippocampal dentate gyrus in mice deficient in PDE4D (4DKO) with or without rolipram (Rol) treatment. a, b, Confocal micrographs (a) and quantification (b) of BrdU-labeled cells (green) in the dentate gyrus from 4DKO or WT mice with or without rolipram. The majority of BrdU-labeled cells were located in the SGZ and hilus (H). While 4DKO and rolipram-treated WT mice displayed increases in BrdU-positive cells, rolipram did not alter BrdU-positive cells in 4DKO mice. c, d, Phenotype of BrdU-positive cells in the dentate gyri of 4DKO mice. Confocal micrographs of cells double-labeled for BrdU (green; left) and the neuronal marker NeuN (red; middle and top) or the glial marker S100β (blue; middle and bottom). e, Unaltered percentages of neuronal and glial cells labeled by BrdU in the dentate gyri of 4DKO mice with or without rolipram treatment. f, Representative confocal micrographs of cells triple-labeled for BrdU, the newborn neuron marker PSA-NCAM (red), and pCREB (blue) in the dentate gyri of 4DKO mice, which indicate colocalization of PSA-NCAM and pCREB in developing BrdU-positive cells. g, Unaltered percentages of newborn neurons labeled by PSA-NCAM in BrdU-positive cells in the dentate gyrus from WT or 4DKO mice following rolipram or vehicle treatment. PSA-NCAM-labeled cells comprised 84.5% of BrdU-positive cells. h, Unaltered percentages of pCREB-labeled cells in newborn neurons in the dentate gyrus from WT or 4DKO mice following rolipram or vehicle treatment. pCREB-labeled cells comprised 91.5% of cells double labeled for PSA-NCAM and BrdU. Mice were given rolipram (1.25 mg/kg, i.p.) or vehicle once daily for 26 d; BrdU (100 mg/kg) was injected (i.p.) once daily on days 10, 12, and 14. On day 26, 1 h after the rolipram injection, mice were perfused and frozen brain sections prepared for immunohistochemistry. Bars shown are means ± SEM; n = 4; *p < 0.05 versus WT plus vehicle (Veh).
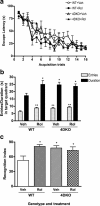
Figure 5.
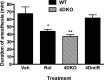
Effects of PDE4D deficiency on levels of pCREB and Sox2 in the hippocampus in the presence or absence of rolipram (Rol). a, Representative confocal micrographs of pCREB-positive cells (blue) in the dentate gyrus from mice deficient in PDE4D (4DKO) or WT controls following chronic treatment with rolipram or vehicle. b, c, Changes in expression of pCREB (b) and Sox2 (c) in the hippocampus from WT or 4DKO mice treated repeatedly with rolipram or vehicle. Bottom panels are representative immunoblots of pCREB or Sox2 detected by Western blotting; top panels are the corresponding quantification. Whereas 4DKO and rolipram-treated WT mice displayed increases in pCREB-positive cells and expression of pCREB and Sox2, the effects of PDE4D deficiency were not altered by chronic rolipram treatment. Rolipram (1.25 mg/kg) was injected (i.p.) once a day for 26 d; 1 h after the last injection, mice were killed and the brains prepared for immunohistochemistry or hippocampi were dissected for immunoblot analysis. Bars shown are means ± SEM; n = 4; *p < 0.05 versus corresponding WT plus vehicle (Veh).
Figure 6.
Effects of the lenti-PDE4D-miRNA (4DmiR) on expression of PDE4 splice variants in the mouse hippocampus, with or without rolipram (Rol) treatment. a, b, Representative micrographs of injection sites in the hippocampus (left) and expression of NC (a) and 4DmiR (b), indicated by EGFP (green; middle and right panels; the right panels are magnifications of the middle panels), in the dentate gyrus 16 d following bilateral microinfusions of the lentiviral vectors into the dentate gyri (4 × 106 TU/μl, 1 μl per side). c, Representative immunoblots of PDE4D3, 4D4, 4D5, 4A5, 4B1, and 4B4 detected by Western blotting using punched hippocampal tissues (3 mm in diameter surrounding the injection hole in the hippocampus) from mice treated with NC or 4DmiR, with or without rolipram treatment (1.25 mg/kg) that was injected (i.p.) once daily for 16 d. d, e, Quantification of PDE4D3–5 (d) and PDE4A/B (e) from the respective immunoblots (c). Bars shown are means ± SEM; n = 4; *p < 0.05, **p < 0.01 versus corresponding NC plus vehicle (Veh); $p < 0.05 versus corresponding 4DmiR plus vehicle.
Figure 7.
Effects of lenti-PDE4D-miRNAs (4DmiR) on memory and hippocampal pCREB and neurogenesis, with or without rolipram (Rol) treatment in mice. a, b, Memory performance of mice treated with 4DmiR or NC alone or in combination with rolipram in the object recognition test (a) and step-down passive avoidance test (b). 4DmiR or rolipram alone enhanced long-term memory, as indicated by increased recognition index and retention latency, respectively, tested 24 h after training. c, Increased expression of pCREB in the hippocampus from mice treated with 4DmiR or rolipram alone or in combination. Bottom panels are representative immunoblots of pCREB detected by Western blotting; top panels are quantification. d, e, Confocal micrographs (d) and quantification (e) of BrdU-labeled cells (red) in the dentate gyrus from mice treated with 4DmiR or NC with or without rolipram treatment. BrdU (100 mg/kg) was injected (i.p.) three times with 3 h intervals. Twenty-four hours after the first injection of BrdU, 4DmiR or NC was microinfused into bilateral dentate gyri using a single dose of 4 × 106 TU/μl/side, after which rolipram (1.25 mg/kg) or its vehicle was injected (i.p.) once daily for 12 or 14 d before the object recognition or passive avoidance test, respectively. One hour after the injection of rolipram on day 16, mice were killed and their brains prepared for immunohistochemistry or their hippocampi dissected and punched for immunoblot analysis. Bars are means ± SEM; n = 8–10 (a,b) or 4 (c–e); *p < 0.05, ** p < 0.01 versus corresponding NC plus vehicle (Veh). H, Hilus.
Figure 8.
Mice deficient in PDE4D (4DKO) or treated with rolipram (Rol) or 4DmiRs displayed different effects on xylazine/ketamine-induced anesthesia. Lentiviral 4DmiR (4 × 106 TU/μl/side) was infused into bilateral dentate gyri 2 weeks before the test; xylazine (10 mg/kg) and ketamine (80 mg/kg) were injected (i.p.) 15 min after rolipram (1 mg/kg) or vehicle. Once animals became ataxic, they were placed on their backs until they righted themselves three times within 30 s. The duration of anesthesia was determined as the time between the loss and return of the righting reflex. Bars are means ± SEM; n = 10–16; *p < 0.05, **p < 0.01 versus vehicle (Veh).
Similar articles
- RNA interference-mediated knockdown of long-form phosphodiesterase-4D (PDE4D) enzyme reverses amyloid-β42-induced memory deficits in mice.
Zhang C, Cheng Y, Wang H, Wang C, Wilson SP, Xu J, Zhang HT. Zhang C, et al. J Alzheimers Dis. 2014;38(2):269-80. doi: 10.3233/JAD-122236. J Alzheimers Dis. 2014. PMID: 23948935 - RNA interference-mediated phosphodiesterase 4D splice variants knock-down in the prefrontal cortex produces antidepressant-like and cognition-enhancing effects.
Wang ZZ, Zhang Y, Liu YQ, Zhao N, Zhang YZ, Yuan L, An L, Li J, Wang XY, Qin JJ, Wilson SP, O'Donnell JM, Zhang HT, Li YF. Wang ZZ, et al. Br J Pharmacol. 2013 Feb;168(4):1001-14. doi: 10.1111/j.1476-5381.2012.02225.x. Br J Pharmacol. 2013. PMID: 23003922 Free PMC article. - Phosphodiesterase-4D Knock-down in the Prefrontal Cortex Alleviates Chronic Unpredictable Stress-Induced Depressive-Like Behaviors and Memory Deficits in Mice.
Wang ZZ, Yang WX, Zhang Y, Zhao N, Zhang YZ, Liu YQ, Xu Y, Wilson SP, O'Donnell JM, Zhang HT, Li YF. Wang ZZ, et al. Sci Rep. 2015 Jul 10;5:11332. doi: 10.1038/srep11332. Sci Rep. 2015. PMID: 26161529 Free PMC article. - Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs.
Zhang HT. Zhang HT. Curr Pharm Des. 2009;15(14):1688-98. doi: 10.2174/138161209788168092. Curr Pharm Des. 2009. PMID: 19442182 Review. - Research progress on phosphodiesterase 4 inhibitors in central nervous system diseases.
Adili A, Dilihumaer A, Zhu H, Tang H. Adili A, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jun 6;53(3):390-398. doi: 10.3724/zdxbyxb-2024-0023. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38860393 Free PMC article. Review. Chinese, English.
Cited by
- Inhibition of Phosphodiesterase 4 Suppresses Neuronal Ferroptosis After Cerebral Ischemia/Reperfusion.
Chen K, Xu B, Long L, Wen H, Zhao Q, Tu X, Wang J, Xu J, Wang H. Chen K, et al. Mol Neurobiol. 2024 Sep 17. doi: 10.1007/s12035-024-04495-9. Online ahead of print. Mol Neurobiol. 2024. PMID: 39287745 - Phosphodiesterase 4D activity in acrodysostosis-associated neural pathology: too much or too little?
Gardner OFW, Bai T, Baillie GS, Ferretti P. Gardner OFW, et al. Brain Commun. 2024 Jun 29;6(4):fcae225. doi: 10.1093/braincomms/fcae225. eCollection 2024. Brain Commun. 2024. PMID: 38983619 Free PMC article. Review. - Allosteric inhibition of phosphodiesterase 4D induces biphasic memory-enhancing effects associated with learning-activated signaling pathways.
Jino K, Miyamoto K, Kanbara T, Unemura C, Horiguchi N, Ago Y. Jino K, et al. Psychopharmacology (Berl). 2024 Apr;241(4):805-816. doi: 10.1007/s00213-023-06510-8. Epub 2023 Dec 19. Psychopharmacology (Berl). 2024. PMID: 38114603 - Phosphodiesterase 4 inhibition after retrieval switches the memory fate favoring extinction instead of reconsolidation.
Machado Batista Sohn J, Cardoso NC, Raymundi AM, Prickaerts J, Stern CAJ. Machado Batista Sohn J, et al. Sci Rep. 2023 Nov 21;13(1):20384. doi: 10.1038/s41598-023-47717-1. Sci Rep. 2023. PMID: 37990053 Free PMC article. - Phosphodiesterase and psychiatric disorders: a two-sample Mendelian randomization study.
Jiang M, Yan W, Zhang Y, Lu Z, Lu T, Zhang D, Li J, Wang L. Jiang M, et al. J Transl Med. 2023 Aug 21;21(1):560. doi: 10.1186/s12967-023-04368-0. J Transl Med. 2023. PMID: 37605207 Free PMC article.
References
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev. 1998;26:360–378. - PubMed
- Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets. 2003;7:101–114. - PubMed
- Bolger GB, McPhee I, Houslay MD. Alternative splicing of cAMP-specific phosphodiesterase mRNA transcripts: characterization of a novel tissue-specific isoform, RNPDE4A8. J Biol Chem. 1996;271:1065–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AG031687-02/AG/NIA NIH HHS/United States
- R21 AG031687/AG/NIA NIH HHS/United States
- MH040697/MH/NIMH NIH HHS/United States
- MH051175/MH/NIMH NIH HHS/United States
- R01 MH051175/MH/NIMH NIH HHS/United States
- AG031687/AG/NIA NIH HHS/United States
- R21 AG031687-01A1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials