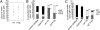Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential - PubMed (original) (raw)
Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential
Momoko Yoshimoto et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The majority of B lymphocytes in the adult mouse are generated in the bone marrow from hematopoietic stem cells (HSCs) that first appear in the aorta-gonado-mesonephros region of the fetus on embryonic day (E) 10.5-11. Comparatively less is known about B-cell development during embryogenesis. For example, which specific embryonic tissues participate in B lymphopoiesis and whether hematopoietic differentiation is skewed toward specific B-cell subsets in the embryo are unanswered questions, because the systemic circulation is initiated early during embryogenesis, resulting in an admixture of cells potentially originating from multiple sites. We demonstrate, using Ncx1(-/-) mice that lack systemic blood circulation, that the E9 yolk sac (YS) and the intra-embryonic para-aortic splanchnopleura (P-Sp) tissues independently give rise to AA4.1(+)CD19(+)B220(lo-neg) B progenitor cells that preferentially differentiate into innate type B-1 and marginal zone (MZ) B cells but not into B-2 cells upon transplantation. We have further demonstrated that these B-1 progenitor cells arise directly from YS and P-Sp hemogenic endothelium. These results document the initial wave of innate B lymphopoietic progenitor cells available for seeding the fetal liver at E11. The results of these studies expand our knowledge of hemogenic endothelial sites specifying distinct B-1 and MZ cell fates apart from B-2 cells and independent of an HSC origin during development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Representative phenotype of peritoneal and spleen cells in mice reconstituted with WT E9.5 YS and P-Sp cells. (A) Phenotypic analysis of B-1 and B-2 cells in the peritoneal cavity of nontransplanted control C57BL/6 mice (Top) and recipients of YS (Middle)- and P-Sp (Bottom)–derived cells. (B) Phenotypic analysis of B-1, FO B-2, and MZ cells in the spleen of nontransplanted control C57BL/6 mice (Top) and recipients of YS (Middle) and P-Sp (Bottom) cells. Lymphoid gate was determined from the forward scatter (FSC)/ side scatter (SSC) panel of WT BL/6 control peritoneal cells. The E9.5 WT YS and P-Sp cells were injected into 150 cGy-irradiated NOG neonates immediately after isolation. The number of animals examined in each group is listed in
Table S1
.
Fig. 2.
Progenitors that reconstitute B-1 and MZ cells are present in YS and P-Sp. The E9.5 WT YS and P-Sp cells were injected into 150 cGy-irradiated NOG neonates immediately after isolation. (A) The level of donor chimerism in the peritoneal cavity of recipient mice (CD45.1+) that received YS- and P-Sp–derived cells (CD45.2+) is depicted. (B) B-cell subsets within donor IgM+ cells in the peritoneal cavity of recipient mice. The percentage of B-1 cells in the peritoneal cavity of YS or P-Sp reconstituted mice was significantly higher than that observed in the peritoneum of nontransplanted adult C57BL/6 mice or E15.5 fetal liver reconstituted mice (P < 0.05). The percentage of B-2 cells in the peritoneal cavity of YS and P-Sp reconstituted animals was significantly less than that observed in the peritoneum of nontransplanted adult control mice or the E15.5 fetal liver reconstituted mice (P < 0.01). The IgMlowIgDhigh or IgMlowCD23high cells were defined as B-2 cells. (C) B-cell subsets within donor IgM+ cells in the spleen. In addition to B-1 cells, the spleen of mice reconstituted with YS and P-Sp cells also contained donor-derived MZ cells. The percentage of FO cells in the spleen of YS and P-Sp transplanted animals was significantly less than nontransplanted control C57BL/6 mice or fetal liver reconstituted mice (P < 0.01). The percentage of MZ cells in the spleen of YS and P-Sp transplanted mice was significantly higher than nontransplanted control mice or E15.5 fetal liver reconstituted mice (P < 0.05). The number of animals examined in each group is listed in
Table S1
.
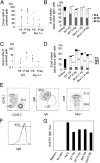
Fig. 3.
E9.0–9.5 Ncx1−/− YS and P-Sp can produce B-1 progenitor cells in vitro. (A) Cells from YS and P-Sp were isolated and placed in culture on OP9 stromal cells as described (19). At day 8, YS and P-Sp cells from WT and Ncx1−/− mice generated AA4.1+CD19+B220lo-neg B-1 progenitor cells. Cells with an AA4.1+CD19−B220+ B-2 progenitor phenotype were not detected. At day 12, the cultured cells became CD19+B220+. (B) Genotyping of cells from Ncx1−/− YS cocultures (lanes 1–3) and control for _Ncx1_−/−, Ncx1+/−, and WT embryos. All mutants express a Lac z transgene. (C) Cobblestone appearance of cells cultured for 12 d. An image of Ncx1−/− YS cell culture is depicted (200×). (D) E8.25 (four to six sp) YS and P-Sp cells can generate AA4.1+CD19+B220lo-neg cells in OP9 culture (representative of three independent experiments). Cell analysis was performed 8 d after culture initiation.
Fig. 4.
AA4.1+CD19+B220+ cells derived from Ncx1−/− YS and P-Sp are functional. E9-9.5 WT and _Ncx1_−/− YS or P-Sp were cultured in vitro, and 12 d later AA4.1+CD19+B220+ cells from the culture were injected into neonatal NOG mice. Recipients were examined 5 wk to 6 mo later (data presented are from 10 to 12 wk after injection). (A) The percentage of WT and Ncx1−/− donor YS- and P-Sp–derived cells in the recipient peritoneal cavity. (B) B-cell subsets within donor IgM+ cells in the peritoneal cavity. WT and Ncx1−/− YS- and P-Sp–derived cells reconstituted B-1 cells and a few B-2 cells (P < 0.01) in the peritoneal cavity. (C) The percentage of WT and Ncx1−/− donor YS- and P-Sp–derived cells in the recipient spleen. (D) B-cell subsets within donor IgM+ cells in the spleen. WT and Ncx1−/− YS- and P-Sp–derived cells primarily reconstituted MZ (P < 0.01) and B-1 cells, but not FO B-2 B cells (P < 0.01) in the spleen. Peritoneal (B) and spleen (D) cells from nontransplanted adult C57BL/6 mice are included as controls. (E) Peritoneal cells were harvested from the primary recipient NOG mice that had been reconstituted 4 mo previously with AA4.1+CD19+B220+ cells (CD45.2+) derived from E9.5 WT YS and P-Sp that emerged in vitro. Cells were then injected into the peritoneal cavity of 300 cGy-irradiated secondary adult NOG recipients (CD45.1+). Two to 4 mo after secondary transplantation, peritoneal cells were analyzed. Donor (CD45.2+ YS or P-Sp)-derived cells were detected as IgMhighIgDlowCD5+B-1a and IgMhighIgDlowCD5− B-1b cells in the secondary recipient animals (YS-derived cell transplanted: n = 3; P-Sp–derived cell transplanted: n = 4). Representative FACS plots are depicted. (F) Cells in the culture expressed surface IgM+ and were expanded upon antigen stimulation with PC to (G) secrete anti-PC–specific IgM antibodies in culture supernatants that were detected by ELISA (representative data from three experiments). PerC: peritoneal cells from C57BL/6 mouse as a positive control.
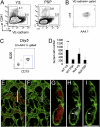
Fig. 5.
B-1 B progenitor cells are derived from VE-cadherin+CD41− hemogenic endothelial cells. (A) VE-cadherin+CD41− (endothelial) cells or VE-cadherin−CD41+ (hematopoietic) cells were sorted from E9.5 WT and _Ncx1_−/− YS and P-Sp cells and plated on OP9 with IL7 and flt-3 ligand. WT FACS dot plots are depicted. _Ncx1_−/− embryos displayed a similar phenotype. (B) All VE-cadherin+CD41− cells coexpressed AA4.1 and c-kit (n = 3). (C) After 5 d of culture, VE-cadherin+CD41− cells from _Ncx1_−/− YS produced AA4.1+CD19+B220dim cells in vitro (representative of four experiments). (D) The number of VE-cadherin+ cells obtained from one embryo equivalent (1 e.e.) is depicted. WT YS VE-cad+ cell number was significantly higher than WT P-Sp cell number (P < 0.01). (E_–_I) Emergence of CD19+ (white, white arrow) cells was detected as a small regionalized population of hematopoietic cells (red) within the E9.0 YS VE-cadherin+ vasculature (green). (E) Image of the E9.0 yolk sac showing hematopoietic cells (red) within the VE-cadherin+ (green) vasculature. (F) Same region as in E depicting small CD19dim (white) population. (G) Orthogonal α-projections (4× zoom) of the region indicated by the yellow line in E. (h and I) Arrows indicate rare CD19+ (white) VE-cadherin (green) double-positive cells. (I) VE-cad+ cells (green) are highlighted. (Scale bar: 100 μm in E and F and 25 μm in G_–_I.)
Fig. 6.
Suggested origin of B-1 progenitor cells in the mouse embryo. B-1 progenitor cells emerge from YS and P-Sp hemogenic endothelium at E9–9.5. These cells then migrate into the fetal liver and mature into AA4.1+CD19+B220+ cells that can differentiate into B-1 and MZ cells. HSCs subsequently emerge at E10.5 in the AGM region and migrate into the fetal liver where they differentiate into AA4.1+CD19+B220+ cells that are primarily the precursors of B-2 cells. We thus propose that the AA4.1+CD19+B220+ population in the E11 fetal liver is developmentally heterogeneous. A proportion of these cells is independently derived from YS and P-Sp sites that generate B-1 and MZ B cells. Other B progenitor cells are primarily HSC-derived and will generate B-2 cells.
Similar articles
- LYVE1 Marks the Divergence of Yolk Sac Definitive Hemogenic Endothelium from the Primitive Erythroid Lineage.
Lee LK, Ghorbanian Y, Wang W, Wang Y, Kim YJ, Weissman IL, Inlay MA, Mikkola HKA. Lee LK, et al. Cell Rep. 2016 Nov 22;17(9):2286-2298. doi: 10.1016/j.celrep.2016.10.080. Cell Rep. 2016. PMID: 27880904 Free PMC article. - Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence.
Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH, Yoder MC. Yoshimoto M, et al. Blood. 2012 Jun 14;119(24):5706-14. doi: 10.1182/blood-2011-12-397489. Epub 2012 Mar 19. Blood. 2012. PMID: 22431573 Free PMC article. - Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen.
Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Ghosn EE, et al. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2879-84. doi: 10.1073/pnas.1019764108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282663 Free PMC article. - Embryonic hematopoiesis.
Golub R, Cumano A. Golub R, et al. Blood Cells Mol Dis. 2013 Dec;51(4):226-31. doi: 10.1016/j.bcmd.2013.08.004. Epub 2013 Sep 13. Blood Cells Mol Dis. 2013. PMID: 24041595 Review. - The first wave of B lymphopoiesis develops independently of stem cells in the murine embryo.
Yoshimoto M. Yoshimoto M. Ann N Y Acad Sci. 2015 Dec;1362:16-22. doi: 10.1111/nyas.12612. Epub 2015 Feb 26. Ann N Y Acad Sci. 2015. PMID: 25721392 Review.
Cited by
- Decoding the human prenatal immune system with single-cell multi-omics.
Haniffa M, Maartens A, Winheim E, Jardine L. Haniffa M, et al. Nat Rev Immunol. 2024 Oct 31. doi: 10.1038/s41577-024-01099-1. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39482372 Review. - The immunology of B-1 cells: from development to aging.
Mattos MS, Vandendriessche S, Waisman A, Marques PE. Mattos MS, et al. Immun Ageing. 2024 Aug 2;21(1):54. doi: 10.1186/s12979-024-00455-y. Immun Ageing. 2024. PMID: 39095816 Free PMC article. Review. - Transition of signal requirement in hematopoietic stem cell development from hemogenic endothelial cells.
Morino-Koga S, Tsuruda M, Zhao X, Oshiro S, Yokomizo T, Yamane M, Tanigawa S, Miike K, Usuki S, Yasunaga KI, Nishinakamura R, Suda T, Ogawa M. Morino-Koga S, et al. Proc Natl Acad Sci U S A. 2024 Jul 30;121(31):e2404193121. doi: 10.1073/pnas.2404193121. Epub 2024 Jul 23. Proc Natl Acad Sci U S A. 2024. PMID: 39042698 - New insights into the endothelial origin of hematopoietic system inspired by "TIF" approaches.
Hou S, Guo X, Du J, Ding X, Ning X, Wang H, Chen H, Liu B, Lan Y. Hou S, et al. Blood Sci. 2024 Jul 16;6(4):e00199. doi: 10.1097/BS9.0000000000000199. eCollection 2024 Oct. Blood Sci. 2024. PMID: 39027902 Free PMC article. Review.
References
- Weissman IL, Baird S, Gardner RL, Papaioannou VE, Raschke W. Normal and neoplastic maturation of T-lineage lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41:9–21. - PubMed
- Weissman IL, Papaioannou V, Gardner R. Differentiation of Normal and Neoplastic Hematopoietic Cells. Vol. 5. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1978. Fetal hematopoietic origin of the adult hematolymphoid system; pp. 33–47.
- Moore MA, Metcalf D. Ontogeny of the haemopoietic system: Yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. - PubMed
- Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI080759/AI/NIAID NIH HHS/United States
- R01 AI021256/AI/NIAID NIH HHS/United States
- R01 AI080759/AI/NIAID NIH HHS/United States
- AI21256/AI/NIAID NIH HHS/United States
- R37 AI021256/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous