The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism - PubMed (original) (raw)
The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism
Chunxing Yang et al. PLoS One. 2010.
Abstract
TAR DNA binding protein 43 KD (TDP-43) is an essential gene that regulates gene transcription, mRNA splicing and stability. In amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), two fatal neurodegenerative diseases, TDP-43 is fragmented, generating multiple fragments that include the C-terminal fragment of ∼25 KD. The role of these fragments in the pathogenesis of ALS and FTD is not clear. Here we investigated the aggregation propensity in various polypeptide regions of TDP-43 in mammalian cells and the effect of these fragments on cultured neurons. By expressing the full length and various TDP-43 fragments in motor neuron-derived NSC-34 cells and primary neurons, we found that both N- and C-terminal fragments of TDP-43 are prone to aggregate and the C-terminal end of RRM2 region is required, though not sufficient, for aggregation. The aggregation of the TDP-43 fragments can drive co-aggregation with the full-length TDP-43, consequently reducing the nuclear TDP-43. In addition, the TDP-43 fragments can impair neurite growth during neuronal differentiation. Importantly, overexpression of the full-length TDP-43 rescues the neurite growth phenotype whereas knockdown of the endogenous TDP-43 reproduces this phenotype. These results suggest that TDP-43 fragments, particularly the pathologically relevant C-terminal fragments, can impair neuronal differentiation by dominant-negatively interfering with the function of the full length TDP-43, thus playing a role in pathogenesis in ALS and FTD.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Constructs of TDP-43 fragments.
A schematic illustration of various constructs of human TDP-43 fragments. The protein domains are marked on the top. RRM means RNA recognition motif. GRD means glycine-rich domain. The structure is not drawn in proportion. The amino acid numbers are marked for each mutant. The red segment in construct 4 marks the C-terminal half of the RRM2 that is necessary for aggregation (see text). For construct 8, the GRD was deleted and then the two fragments were joined together. EGFP was fused to the C-terminus of all the TDP-43 constructs.
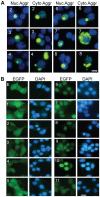
Figure 2. TDP-43 aggregation and distribution of non-aggregated TDP-43-EGFP fragments in NSC-34 cells.
(A) Examples of cells with TDP-43 aggregates. The numbers in each row indicate the constructs. Both nuclear (Nuc Aggr) and cytoplasmic aggregates (Cyto Aggr) are shown. The nuclei are visualized with DAPI. The scale bar marks 10 µm. (B) TDP-43-EGFP distribution in cells without aggregates. The cells were transfected with the constructs: EGFP (E) and various TDP-43-EGFP as marked on each row of panels. The cells were fixed 48 hrs after the transfection and then photographed.
Figure 3. Expression of the various TDP-43-EGFP constructs in NSC-34 cells.
(A) Forty eight hours after transfection, proteins were extracted from NSC-34 cells and the fusion proteins were detected by Western blot using an EGFP antibody. Fifteen µg of protein was loaded in each lane. α-Tubulin was detected as loading control. Left and right panels are two independent gels. (B) Cells was transfected with construct 1 alone and then stained with antiTDP-43 primary and Alexa Fluor®568-conjugated secondary antibodies. The nuclei are stained with DAPI. The scale bar marks 10 µm.
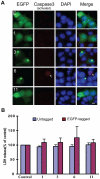
Figure 4. TDP-43 fragments did not increase cell death.
(A) Caspase 3 activation was not associated with the expression of TDP-43 fragments. NSC-34 cells were transfected with the EGFP-tagged constructs (see Fig. 1) for 72 hrs. The cells were fixed and stained for activated caspase 3 (red). The numbers in each row indicate the constructs. DAPI stain marks the nucleus. The scale bar represents 10 µm. Notice that cells with caspase 3 activation could be observed (arrow) but they were not preferentially associated with the transfected cells. (B) LDH release was not significantly elevated in NSC-34 cells transfected with the TDP-43 fragments. Both untagged and EGFP-tagged TDP-43 constructs were transfected into NSC34 cells for 72 hrs. LDH activity was measured in the media and normalized to the activity in the carrier vector transfected control cells (set at 100%). The averages from three experiments were plotted. Error bars represent standard deviation. The numbers along the X-axis indicate the constructs.
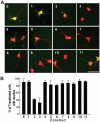
Figure 5. Expression of TDP-43 fragments inhibits neurite growth in motor neuron-like NSC-34 cells.
(A) The cells were transfected with EGFP and each of the TDP-43 constructs as shown in Fig. 1. The cells were also transfected with tdTomato to help visualizing neurites. Twenty-four hours after the transfection, the cells were differentiated to neurons by serum withdraw and cultured for another 72 hours before being fixed and photographed. (B) TDP-43-EGFP fragments were transfected with either tdTomato (white and black bars) or with the full-length TDP-43 tagged with tdTomato (green and red bars; also see Fig. 9). Percent of cells bearing neurites were quantified and averaged from three experiments. Error bars represent standard error. Statistics using one-factor ANOVA showed significant difference among the cells transfected with different constructs (F = 24.6, p = 2×10−27). Tukey's Post Hoc Test showed significant differences comparing the EGFP-transfected cells (E) with the white bars of constructs 2, 3, 4 and 5 and the black bars of constructs 2, 3, 4, 6, 7 and 8 with (p<0.01).
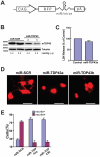
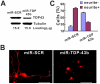
Figure 6. Knockdown of TDP43 inhibits neurite growth during neuronal differentiation of NSC-34 cells.
(A) The miRNA expression construct, which uses the CAG promoter to drive expression of RFP (dsRed2) and a miRNA in an intron placed in the 3′-UTR of the RFP gene. The miRNA targets mouse TDP-43. (B) Western blot of proteins extracted from the transfected NSC-34 cells. TDP-43 was knocked down in cells transfected with two different miRNA constructs. Tubulin was detected as a loading control. miR-SCR is a scrambled miRNA that does not target any specific genes. (C) Knockdown TDP43 does not increase cell death. Scrambled or miR-TDP43a constructs were transfected into NSC34 cells. LDH activity was assayed in the media was 72 hrs after transfection. (D) Knockdown of TDP43 inhibits neurite growth in NSC34 cells. NSC34 cells were transfected with the miRNA constructs. Forty-eight hours later, neuronal differentiation was initiated. After another 72 hours, the cells were fixed and visualized by fluorescence microscopy. Scale bar represents 20 µm. (E) Quantification of cells with or without neurites. Results are shown as average percentage of cells from three independent experiments. Error bars are standard error. Statistics using one-factor ANOVA indicated significant difference among the cells transfected with different miRNA constructs (p = 0.0001). Tukey's Post Hoc Test showed significant differences comparing the miR-SRC-transfected cells with the cells transfected with either miR-TDP43s (p<0.01).
Figure 7. Expression of TDP-43 fragments inhibits neurite growth in primary rat forebrain neurons.
(A) The rat forebrain neural precursors were transfected with EGFP and each of the TDP-43 constructs as shown in Fig. 1. The cells were also transfected with tdTomato for visualizing neurites. Twenty-four hours after the transfection, the cells were differentiated to neurons by withdrawing basic fibroblast growth factor 2 from the medium and cultured for another 72 hours before being fixed and photographed. (B) Quantification of neurite-bearing cells as shown in (A). Values are averages of four independent experiments. Error bars represent standard error.
Figure 8. Knockdown of TDP43 inhibits neurite growth in differentiated primary rat fore brain neurons.
(A) Western blot of proteins extracted from the transfected Rat-2 cells. TDP-43 was knocked down in cells transfected with miR-TDP43b. α-Tubulin was detected as a loading control. (B) Knockdown of TDP43 inhibits neurite growth in rat fore brain neurons. The cells were transfected with the miRNA construct for 48 hours and then differentiated. After another 72 hours, the cells were fixed and visualized by fluorescence microscopy. Scrambled miRNA (miR-SCR) was the control. Scale bar represents 100 µm. (C) Quantification of cells with or without neurites. Results are shown as average percentage of cells from three independent experiments. Error bars are standard error. Statistics using one-factor ANOVA indicated significant difference between the cells transfected with miR-SCR and those with miR-TDP43b (p = 0.00002).
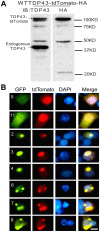
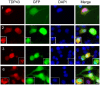
Figure 9. Coaggregation of wild type TDP-43 with its fragments.
(A) The full-length TDP-43-tdTomato construct expressed the fusion protein. Proteins were extracted 48 hrs after the transfection of NSC-34 cells. Fifteen µg of the protein was resolved by SDS-PAGE and blotted. TDP-43-tdTomato was detected using a TDP43 antibody or an HA-tag antibody. (B) The EGFP-tagged, aggregation-prone TDP-43 fragments (2, 3, 4, 6, 7 and 8) were cotransfected with the full-length TDP-43-tdTomato construct. The full-length TDP-43 was colocalized with all of these mutants in aggregates. Two mutants (5 and 11) that do not form aggregates were also cotransfected with the full-length TDP-43. In these cells, the mutants (green) were diffusely distributed throughout the cells and the full-length wild type (red) was predominantly in the nucleus.
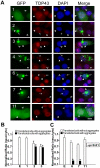
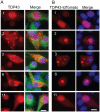
Figure 10. Expression of truncated TDP43 proteins reduces endogenous TDP-43 in the nucleus.
NSC-34 cells were transfected with the constructs expressing the EGFP-tagged full length and truncated TDP-43 (see Fig. 1) as indicated by the numbers in each row of panels. Seventy two hours after the transfection, the cells were fixed and stained with antibodies against the N-terminal (for constructs 1, 2, 3, 4) or C-terminal peptides (for constructs 6, 7 and 11) of TDP-43 to visualize the endogenous TDP-43. The cells with aggregates (green, arrows) showed reduced staining nuclear TDP43 (red, filled arrowheads) compared with the untransfected cells (open arrowheads). Scale bar marks 10 µm. (B) Density ratio of nuclear to cytoplasmic TDP43 in cells stained by the antibody against the N-terminus of TDP-43. The construct 4 in A was not quantified because the number of transfected cells was too few to be reliable. (C) Density ratio of nuclear to cytoplasmic TDP43 in cells stained by the antibody against the C-terminus of TDP-43. The ratios were normalized against the average from the cells transfected by the EGFP construct. Each bar represents values averaged from between 18 to 28 cells. Error bars are standard error. The ratios from the cells transfected with the TDP-43 constructs were compared with the ratio from the cells transfected with the EGFP construct using student's t test with Bonferroni correction. The stars indicate p<0.01. Bars without stars indicate p>0.05.
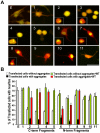
Figure 11. Untagged TDP-43 fragments form aggregates and coaggregate with the full-length TDP-43.
(A) Untagged TDP-43 constructs were transfected into NSC-34 cells for 72 hrs. The cells were fixed and stained with TDP-43 (red) and tubulin (green) antibodies. The nucleus was revealed by DAPI staining. Notice that the construct 1 (full-length) and construct 11 did not form aggregates whereas constructs 2, 3 and 6 formed aggregates. (B) Untagged TDP-43 constructs were co-transfected with the tdTomato-tagged full-length TDP-43 for 72 hrs and then fixed. Notice that constructs 1 and 11 did not alter the distribution of the full-length TDP-43 whereas construct 2, 3, and 6 induced aggregation of the full-length TDP-43 and reduced the diffused staining of TDP-43 in the nucleus.
Figure 12. Untagged TDP-43 impaired neurite growth during neuronal differentiation.
Untagged TDP-43 constructs were cotransfected with EGFP into NSC-34 cells for 24 hrs. The cells were differentiated for 72 hrs and fixed. Notice that cells transfected with the full-length TDP-43 grow long neurites whereas cells transfected with constructs 2 and 3 showed impaired neurite growth regardless whether the cell had aggregates (inserts) or not. Cells transfected with construct 6 showed impaired neurite growth only when aggregates were formed (insert).
Figure 13. Effects of TDP43 fragments on CFTR splicing.
(A) A schematic illustration of the CFTR reporter construct and its splicing products. The large arrow represents site of the promoter, black boxes represent non-CFTR exons, the white box represent CFTR exon, and small arrows depict primers used for amplification . (B) HEK293 cells were transfected with full length or truncated TDP-43 constructs and (TG)13T3 CFTR reporter. The reporter transcript was detected by PCR using the primers indicated in (A). Top and lower bands correspond to the complete exon 9 (the white bar in A) inclusion and exclusion, respectively. The middle band corresponds to the splicing product from a cryptic 3′ splicing site (represented by the dashed line near the 3′ end of the white bar in A). As a control, TDP43 siRNA (siTDP43) was transfected. The numbers on the top of the lane indicate the constructs and E represents EGFP vector only.
Similar articles
- Neurotoxic effects of TDP-43 overexpression in C. elegans.
Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, Petrucelli L, Link CD. Ash PE, et al. Hum Mol Genet. 2010 Aug 15;19(16):3206-18. doi: 10.1093/hmg/ddq230. Epub 2010 Jun 8. Hum Mol Genet. 2010. PMID: 20530643 Free PMC article. - The structural integrity of TDP-43 N-terminus is required for efficient aggregate entrapment and consequent loss of protein function.
Romano V, Quadri Z, Baralle FE, Buratti E. Romano V, et al. Prion. 2015;9(1):1-9. doi: 10.1080/19336896.2015.1011885. Prion. 2015. PMID: 25635624 Free PMC article. - ALS-causing cleavages of TDP-43 abolish its RRM2 structure and unlock CTD for enhanced aggregation and toxicity.
Wei Y, Lim L, Wang L, Song J. Wei Y, et al. Biochem Biophys Res Commun. 2017 Apr 15;485(4):826-831. doi: 10.1016/j.bbrc.2017.02.139. Epub 2017 Feb 28. Biochem Biophys Res Commun. 2017. PMID: 28257838 - The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.
Yamashita T, Kwak S. Yamashita T, et al. Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review. - [The molecular mechanisms of intracellular TDP-43 aggregates].
Nonaka T, Arai T, Hasegawa M. Nonaka T, et al. Brain Nerve. 2009 Nov;61(11):1292-300. Brain Nerve. 2009. PMID: 19938686 Review. Japanese.
Cited by
- Low molecular weight species of TDP-43 generated by abnormal splicing form inclusions in amyotrophic lateral sclerosis and result in motor neuron death.
Xiao S, Sanelli T, Chiang H, Sun Y, Chakrabartty A, Keith J, Rogaeva E, Zinman L, Robertson J. Xiao S, et al. Acta Neuropathol. 2015 Jul;130(1):49-61. doi: 10.1007/s00401-015-1412-5. Epub 2015 Mar 19. Acta Neuropathol. 2015. PMID: 25788357 Free PMC article. - Aggregation-prone TDP-43 sequesters and drives pathological transitions of free nuclear TDP-43.
Keating SS, Bademosi AT, San Gil R, Walker AK. Keating SS, et al. Cell Mol Life Sci. 2023 Mar 17;80(4):95. doi: 10.1007/s00018-023-04739-2. Cell Mol Life Sci. 2023. PMID: 36930291 Free PMC article. - Histone H3 deacetylation promotes host cell viability for efficient infection by Listeria monocytogenes.
Eldridge MJG, Hamon MA. Eldridge MJG, et al. PLoS Pathog. 2021 Dec 20;17(12):e1010173. doi: 10.1371/journal.ppat.1010173. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34929015 Free PMC article. - Structural polymorphism of the low-complexity C-terminal domain of TDP-43 amyloid aggregates revealed by solid-state NMR.
Shenoy J, Lends A, Berbon M, Bilal M, El Mammeri N, Bertoni M, Saad A, Morvan E, Grélard A, Lecomte S, Theillet FX, Buell AK, Kauffmann B, Habenstein B, Loquet A. Shenoy J, et al. Front Mol Biosci. 2023 Mar 29;10:1148302. doi: 10.3389/fmolb.2023.1148302. eCollection 2023. Front Mol Biosci. 2023. PMID: 37065450 Free PMC article. - The N-terminal dimerization is required for TDP-43 splicing activity.
Jiang LL, Xue W, Hong JY, Zhang JT, Li MJ, Yu SN, He JH, Hu HY. Jiang LL, et al. Sci Rep. 2017 Jul 21;7(1):6196. doi: 10.1038/s41598-017-06263-3. Sci Rep. 2017. PMID: 28733604 Free PMC article.
References
- Wang HY, Wang IF, Bose J, Shen CK. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. - PubMed
- Wu LS, Cheng WC, Hou SC, Yan YT, Jiang ST, et al. TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48:56–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous