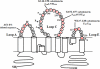Identification of two major conformational aquaporin-4 epitopes for neuromyelitis optica autoantibody binding - PubMed (original) (raw)
Identification of two major conformational aquaporin-4 epitopes for neuromyelitis optica autoantibody binding
Francesco Pisani et al. J Biol Chem. 2011.
Abstract
Neuromyelitis optica (NMO) is an autoimmune demyelinating disease characterized by the presence of anti-aquaporin-4 (AQP4) antibodies in the patient sera. We recently reported that these autoantibodies are able to bind AQP4 when organized in the supramolecular structure called the orthogonal array of particles (OAP). To map the antigenic determinants, we produced a series of AQP4 mutants based on multiple alignment sequence analysis between AQP4 and other OAP-forming AQPs. Mutations were introduced in the three extracellular loops (A, C, and E), and the binding capacity of NMO sera was tested on AQP4 mutants. Results indicate that one group of sera was able to recognize a limited portion of loop C containing the amino acid sequence (146)GVT(T/M)V(150). A second group of sera was characterized by a predominant role of loop A. Deletion of four AQP4-specific amino acids ((61)G(S/T)E(N/K)(64)) in loop A substantially affected the binding of this group of sera. However, the binding capacity was further reduced when amino acids in loop A were mutated together with those in loop E or when those in loop C were mutated in combination with loop E. Finally, a series of AQP0 mutants were produced in which the extracellular loops were progressively changed to make them identical to AQP4. Results showed that none of the mutants was able to reproduce in AQP0 the NMO-IgG epitopes, indicating that the extracellular loop sequence by itself was not sufficient to determine the rearrangement required to create the epitopes. Although our data highlight the complexity of the disease, this study identifies key immunodominant epitopes and provides direct evidence that the transition from AQP4 tetramers to AQP4-OAPs involves conformational changes of the extracellular loops.
Figures
FIGURE 1.
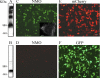
NMO-IgG does not recognize non-OAP-forming M23-AQP4. AQP4 immunoblot was performed after BN-PAGE using M23-mCherry (A) and GFP-M23 (B) protein extracts. Note the presence of multiple bands only in sample extracts from M23-mCherry, indicating the expression of OAPs. Representative immunofluorescence analysis of NMO-IgG binding using M23-mCherry (C) and GFP-M23 cells (D) is shown. Expression of M23-mCherry (red, E) and GFP-M23 (green, F) in transiently transfected HeLa cells is shown. Inset in C shows high magnification of a transfected cells with dot staining. Scale bars, 1 μm and 15 μm for the others.
FIGURE 2.
Comparison of the primary amino acid sequence of rat, mouse, and human AQP4 and AQP0 and of Cicadella AQP. Residues identical in all sequences are indicated by asterisk, and partially conserved amino acids are indicated by a double or single dot. The exposed extracellular loops are highlighted. The amino acid positions are referred to the AQP4-M1 isoform.
FIGURE 3.
Schematic representation of mutations performed in AQP4 extracellular loops. Mutated amino acids, highlighted in red, are flanked by the mutant name and sequence. For more details see also Table 1.
FIGURE 4.
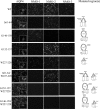
Contribution of loop A, C, and E in the formation of NMO-IgG epitope. Immunofluorescence experiments performed on HeLa cells transfected with mutants are reported in Table 1. Anti-AQP4 antibody (AQP4) and NMO-1, NMO-2, and NMO-3 sera are shown. Scale bars, 20 μm.
FIGURE 5.
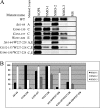
Immunoprecipitation experiment to evaluate the loop A, C, and E contributions into the NMO-IgG epitopes. A, experiment performed with NMO-1, NMO-2, and NMO-3 sera in the AQP4 mutants described previously. Immunoprecipitated proteins were revealed with anti-AQP4 commercially available antibodies. AQP4 antibodies and multiple sclerosis (MS) sera were used as positive and negative control, respectively. B, densitometric analysis of the immunoprecipitated AQP4 described in A (n = 3).
FIGURE 6.
Schematic model on how NMO epitopes could be associated to OAPs formation. Side and top views of an AQP4 tetramer (A) and OAPs are shown (B). A, extracellular loops interactions in the tetramer do not generate the NMO-IgG epitope. B, when AQP4 organizes in OAPs, the extracellular loops of each tetramer rearrange and create at least two different NMO-IgG epitopes.
Similar articles
- Identification of a point mutation impairing the binding between aquaporin-4 and neuromyelitis optica autoantibodies.
Pisani F, Mola MG, Simone L, Rosito S, Alberga D, Mangiatordi GF, Lattanzi G, Nicolotti O, Frigeri A, Svelto M, Nicchia GP. Pisani F, et al. J Biol Chem. 2014 Oct 31;289(44):30578-30589. doi: 10.1074/jbc.M114.582221. Epub 2014 Sep 19. J Biol Chem. 2014. PMID: 25239624 Free PMC article. - Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays.
Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Crane JM, et al. J Biol Chem. 2011 May 6;286(18):16516-24. doi: 10.1074/jbc.M111.227298. Epub 2011 Mar 21. J Biol Chem. 2011. PMID: 21454592 Free PMC article. - Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies.
Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, Lia A, Trojano M, Frigeri A, Svelto M. Nicchia GP, et al. Glia. 2009 Oct;57(13):1363-73. doi: 10.1002/glia.20855. Glia. 2009. PMID: 19229993 - Neuromyelitis optica pathogenesis and aquaporin 4.
Graber DJ, Levy M, Kerr D, Wade WF. Graber DJ, et al. J Neuroinflammation. 2008 May 29;5:22. doi: 10.1186/1742-2094-5-22. J Neuroinflammation. 2008. PMID: 18510734 Free PMC article. Review. - Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO.
Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L. Verkman AS, et al. Brain Pathol. 2013 Nov;23(6):684-95. doi: 10.1111/bpa.12085. Brain Pathol. 2013. PMID: 24118484 Free PMC article. Review.
Cited by
- AQP4-dependent water transport plays a functional role in exercise-induced skeletal muscle adaptations.
Basco D, Blaauw B, Pisani F, Sparaneo A, Nicchia GP, Mola MG, Reggiani C, Svelto M, Frigeri A. Basco D, et al. PLoS One. 2013;8(3):e58712. doi: 10.1371/journal.pone.0058712. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520529 Free PMC article. - Aquaporin-4 in Neuromyelitis Optica Spectrum Disorders: A Target of Autoimmunity in the Central Nervous System.
Abe Y, Yasui M. Abe Y, et al. Biomolecules. 2022 Apr 17;12(4):591. doi: 10.3390/biom12040591. Biomolecules. 2022. PMID: 35454180 Free PMC article. Review. - Aquaporin 4 and neuromyelitis optica.
Papadopoulos MC, Verkman AS. Papadopoulos MC, et al. Lancet Neurol. 2012 Jun;11(6):535-44. doi: 10.1016/S1474-4422(12)70133-3. Epub 2012 May 16. Lancet Neurol. 2012. PMID: 22608667 Free PMC article. Review. - Sera from Patients with NMOSD Reduce the Differentiation Capacity of Precursor Cells in the Central Nervous System.
Gómez-Pinedo U, García-Ávila Y, Gallego-Villarejo L, Matías-Guiu JA, Benito-Martín MS, Esteban-García N, Sanclemente-Alamán I, Pytel V, Moreno-Jiménez L, Sancho-Bielsa F, Vidorreta-Ballesteros L, Montero-Escribano P, Matías-Guiu J. Gómez-Pinedo U, et al. Int J Mol Sci. 2021 May 14;22(10):5192. doi: 10.3390/ijms22105192. Int J Mol Sci. 2021. PMID: 34068922 Free PMC article. - Detection of anti-aquaporin-4 autoantibodies in the sera of Chinese neuromyelitis optica patients.
Li M, Su W, Wang J, Pisani F, Frigeri A, Ma T. Li M, et al. Neural Regen Res. 2013 Mar 15;8(8):708-13. doi: 10.3969/j.issn.1673-5374.2013.08.005. Neural Regen Res. 2013. PMID: 25206717 Free PMC article.
References
- Lennon V. A., Wingerchuk D. M., Kryzer T. J., Pittock S. J., Lucchinetti C. F., Fujihara K., Nakashima I., Weinshenker B. G. (2004) Lancet 364, 2106–2112 - PubMed
- Matsuoka T., Matsushita T., Kawano Y., Osoegawa M., Ochi H., Ishizu T., Minohara M., Kikuchi H., Mihara F., Ohyagi Y., Kira J. (2007) Brain 130, 1206–1223 - PubMed
- Misu T., Fujihara K., Kakita A., Konno H., Nakamura M., Watanabe S., Takahashi T., Nakashima I., Takahashi H., Itoyama Y. (2007) Brain 130, 1224–1234 - PubMed
- Roemer S. F., Parisi J. E., Lennon V. A., Benarroch E. E., Lassmann H., Bruck W., Mandler R. N., Weinshenker B. G., Pittock S. J., Wingerchuk D. M., Lucchinetti C. F. (2007) Brain 130, 1194–1205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases