Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy - PubMed (original) (raw)
Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy
Angela V Hafner et al. Aging (Albany NY). 2010 Dec.
Abstract
Cardiac failure is a leading cause of age-related death, though its root cause remains unknown. Mounting evidence implicates a decline in mitochondrial function due to increased opening of the mitochondrial permeability transition pore (mPTP). Here we report that the NAD+-dependent deacetylase SIRT3 deacetylates the regulatory component of the mPTP, cyclophilin D (CypD) on lysine 166, adjacent to the binding site of cyclosporine A, a CypD inhibitor. Cardiac myocytes from mice lacking SIRT3 exhibit an age-dependent increase in mitochondrial swelling due to increased mPTP opening, a phenotype that is rescued by cyclosporine A. SIRT3 knockout mice show accelerated signs of aging in the heart including cardiac hypertrophy and fibrosis at 13 months of age. SIRT3 knockout mice are also hypersensitive to heart stress induced by transverse aortic constriction (TAC), as evidenced by cardiac hypertrophy, fibrosis, and increased mortality. Together, these data show for the first time that SIRT3 activity is necessary to prevent mitochondrial dysfunction and cardiac hypertrophy during aging and shed light on new pharmacological approaches to delaying aging and treating diseases in cardiac muscle and possibly other post-mitotic tissues.
Conflict of interest statement
D. Sinclair is a consultant to and inventor on patents licensed to Sirtris, a GlaxoSmithKline company aiming to develop drugs based on sirtuin modulation.
Figures
Figure 1.. SIRT3 prevents mitochondrial permeability transition in cardiac tissue during aging.
Mitochondrial swelling induced by Ca2+. Mitochondria from 3 (A), 6 (B) and 16 months old (C) wt and SIRT3−/− mouse hearts subjected to Ca2+-induced mitochondrial swelling, measured as % decrease in the initial optical density (OD540) in the presence or absence of 1 μM CsA (n=4 per group and age). All error bars show s.e.m.
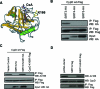
Figure 2.. Cyclophilin D is acetylated at K166
(A) Representative m/z spectrum of peptide TDWLDG-AcK-HVVFGHVK obtained from mass spectrometry analyses of Flag-purified mouse Cyclophilin D. (B) Sequence of Cyclophilin D (Mus musculus). Location of the identified acetylation site. (Peptide: blue; acetylated Lysine: red). (C) Protein sequence alignment of CypD from Homo sapiens, Mus musculus, Danio rerio, Saccharomyces cerevisiae. Lysine 166 is marked in red.
Figure 3.. SIRT3 binds to and deacetylates CypD at K166
(A) CypD-K166 lies adjacent to the CsA binding pocket (Protein Data Bank, 2Z6W). (**B)**Interaction studies using HA-tagged SIRT3, SIRT4 and SIRT5 and FLAG-tagged CypD assessed by coimmunoprecipitation. (C) Specificity of a polyclonal antibody raised against acetylated CypD-K166 confirmed by a lack of Western blot signal for mutant CypD-K166R. SIRT3 and SIRT3-H248Y were co-transfected with FLAG-tagged CypD and the level of acetylation at CypD-K166 was assessed. (D) Vectors for FLAG-tagged SIRT3 and SIRT3-H248Y transfected in HEK 293T cells were immunoprecipitated, then incubated with purified CypD in the presence of the SIRT3 co-substrate NAD+.
Figure 4.. Age- and stress-dependent development of cardiac hypertrophy in SIRT3 −/− mice.
(A) Kaplan-Meier survival plot of 3 months old wt and SIRT3−/− mice after the Transverse Aortic Constriction (TAC) surgery. wt (n=8), SIRT3−/− (n=11). p < 0.05. (B) Representative image and Masson's trichrome staining of hearts from wt and SIRT3−/− mice 4 weeks after the TAC surgery. Heart weight tibia length ratio of wt and SIRT3−/− mice 30 days after the TAC surgery wt (n=8), SIRT3−/− (n=4) mice. (C) Interventricular septal thickness at diastole (Ivs;d) of 2 and 13 month old wt and SIRT3−/− mice after transverse aortic constriction (TAC) for 4 weeks. (D) Representative images of Masson's trichrome staining of transverse sections of heart from 13 month old wt and SIRT3−/− mice. Collagen deposits (fibrosis) stain blue. n=4 mice per group and age (magnification, 20x). **p<0.01. *** p < 0.001. All error bars represent s.e.m.
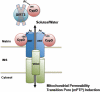
Figure 5.. Regulation of the mPTP by SIRT3
In normal cardiac tissue, SIRT3 targets CypD, maintaining it in a deacetylated state, thus preventing opening of the mPTP during aging and induced cardiac stress. In SIRT3−/− mice, however, CypD is hyperacetylated, resulting in increased induction of the mPTP. A decline in SIRT3 activity over time explains the increase in mitochondrial permeability transition and the decline in cardiac function with age. IMS = inner membrane space.
Comment in
- SIRT3: Striking at the heart of aging.
Liu Y, Zhang D, Chen D. Liu Y, et al. Aging (Albany NY). 2011 Jan;3(1):1-2. doi: 10.18632/aging.100256. Aging (Albany NY). 2011. PMID: 21248372 Free PMC article. No abstract available. - Regulation of the mitochondrial transition pore: impact on mammalian aging.
Osiewacz HD. Osiewacz HD. Aging (Albany NY). 2011 Jan;3(1):10-1. doi: 10.18632/aging.100259. Aging (Albany NY). 2011. PMID: 21248375 Free PMC article. No abstract available. - Sirt3 targets mPTP and prevents aging in the heart.
Sadoshima J. Sadoshima J. Aging (Albany NY). 2011 Jan;3(1):12-3. doi: 10.18632/aging.100266. Aging (Albany NY). 2011. PMID: 21248376 Free PMC article. No abstract available.
Similar articles
- Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore.
Elrod JW, Molkentin JD. Elrod JW, et al. Circ J. 2013;77(5):1111-22. doi: 10.1253/circj.cj-13-0321. Epub 2013 Mar 29. Circ J. 2013. PMID: 23538482 Free PMC article. Review. - Mitochondrial Hyperacetylation in the Failing Hearts of Obese Patients Mediated Partly by a Reduction in SIRT3: The Involvement of the Mitochondrial Permeability Transition Pore.
Castillo EC, Morales JA, Chapoy-Villanueva H, Silva-Platas C, Treviño-Saldaña N, Guerrero-Beltrán CE, Bernal-Ramírez J, Torres-Quintanilla A, García N, Youker K, Torre-Amione G, García-Rivas G. Castillo EC, et al. Cell Physiol Biochem. 2019;53(3):465-479. doi: 10.33594/000000151. Cell Physiol Biochem. 2019. PMID: 31464387 - Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning.
Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM. Hausenloy DJ, et al. Cardiovasc Res. 2010 Oct 1;88(1):67-74. doi: 10.1093/cvr/cvq113. Epub 2010 Apr 16. Cardiovasc Res. 2010. PMID: 20400621 Free PMC article. - SIRT3-Mediated CypD-K166 Deacetylation Alleviates Neuropathic Pain by Improving Mitochondrial Dysfunction and Inhibiting Oxidative Stress.
Yan B, Liu Q, Ding X, Lin Y, Jiao X, Wu Y, Miao H, Zhou C. Yan B, et al. Oxid Med Cell Longev. 2022 Sep 1;2022:4722647. doi: 10.1155/2022/4722647. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092157 Free PMC article. - Sirtuin 3 and mitochondrial permeability transition pore (mPTP): A systematic review.
Yang Y, Wang W, Tian Y, Shi J. Yang Y, et al. Mitochondrion. 2022 May;64:103-111. doi: 10.1016/j.mito.2022.03.004. Epub 2022 Mar 25. Mitochondrion. 2022. PMID: 35346868 Review.
Cited by
- Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore.
Elrod JW, Molkentin JD. Elrod JW, et al. Circ J. 2013;77(5):1111-22. doi: 10.1253/circj.cj-13-0321. Epub 2013 Mar 29. Circ J. 2013. PMID: 23538482 Free PMC article. Review. - Friedreich's ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase.
Wagner GR, Pride PM, Babbey CM, Payne RM. Wagner GR, et al. Hum Mol Genet. 2012 Jun 15;21(12):2688-97. doi: 10.1093/hmg/dds095. Epub 2012 Mar 6. Hum Mol Genet. 2012. PMID: 22394676 Free PMC article. - Sirtuins and pyridine nucleotides.
Abdellatif M. Abdellatif M. Circ Res. 2012 Aug 17;111(5):642-56. doi: 10.1161/CIRCRESAHA.111.246546. Circ Res. 2012. PMID: 22904043 Free PMC article. Review. - Mitochondrial Metabolism in Aging Heart.
Lesnefsky EJ, Chen Q, Hoppel CL. Lesnefsky EJ, et al. Circ Res. 2016 May 13;118(10):1593-611. doi: 10.1161/CIRCRESAHA.116.307505. Circ Res. 2016. PMID: 27174952 Free PMC article. Review. - Quality control systems in cardiac aging.
Quarles EK, Dai DF, Tocchi A, Basisty N, Gitari L, Rabinovitch PS. Quarles EK, et al. Ageing Res Rev. 2015 Sep;23(Pt A):101-15. doi: 10.1016/j.arr.2015.02.003. Epub 2015 Feb 19. Ageing Res Rev. 2015. PMID: 25702865 Free PMC article. Review.
References
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145. - PubMed
- Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575. - PubMed
- Nicholls DG, Budd SL. Neuronal excitotoxicity: the role of mitochondria. Biofactors. 1998;8:287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG028730-01/AG/NIA NIH HHS/United States
- P01 AG027916/AG/NIA NIH HHS/United States
- R01 AG028730/AG/NIA NIH HHS/United States
- R37 AG028730/AG/NIA NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases