Acylideneoxoindoles: a new class of reversible inhibitors of human transglutaminase 2 - PubMed (original) (raw)
Acylideneoxoindoles: a new class of reversible inhibitors of human transglutaminase 2
Cornelius Klöck et al. Bioorg Med Chem Lett. 2011.
Abstract
Inhibitors of human transglutaminase 2 (TG2) are anticipated to be useful in the therapy of a variety of diseases including celiac sprue as well as certain CNS disorders and cancers. A class of 3-acylidene-2-oxoindoles was identified as potent reversible inhibitors of human TG2. Structure-activity relationship analysis of a lead compound led to the generation of several potent, competitive inhibitors. Analogs with significant non-competitive character were also identified, suggesting that the compounds bind at one or more allosteric regulatory sites on this multidomain enzyme. The most active compounds had K(i) values below 1.0 μM in two different kinetic assays for human TG2, and may therefore be suitable for investigations into the role of TG2 in physiology and disease in animals.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Selected TG2 inhibitors – irreversible dipeptide inhibitors (A), irreversible DHI-based inhibitors (B), irreversible DON-based substrate mimics (C), reversible thienopyrimidinones (D), irreversible imidazolium salts (E),, reversible azachalcones (F) and aryl-β-aminoethyl ketones (G, H),
Figure 2
Figure 3
Lineweaver-Burk analysis of the 6-chloro substituted compound 46, confirming its non-competitive inhibitory character. Reversibility was assigned based on the observation that preincubation of TG2 with inhibitors in the presence of 4 mM Ca+2 for 40 min did not appreciably alter the outcome of inhibition experiments (data not shown).
Scheme 1
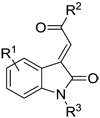
Synthesis of 3-acylidene-2-oxoindoles. Top: Synthesis of N1-H or N1-substituted analogues via condensation-dehydration of N1-H or N1-substituted isatins. Bottom: Synthesis of N1-substituted analogues via N-arylation of N1-H compounds. a) R3X (alkyl bromide or iodide), K2CO3, DMF, 16–48 h. b) acetone, NHEt2, 60 °C, 16 h or aryl methyl ketone, NHEt2, EtOH, RT, 2–48 h. c) HCl, AcOH, reflux, 0.5 h or HCl, AcOH, RT, 16 h. d) PhB(OH)2, CuSO4·5H2O, pyridine, DCM, 16 h.
Similar articles
- An unprecedented dual antagonist and agonist of human Transglutaminase 2.
Yi MC, Palanski BA, Quintero SA, Plugis NM, Khosla C. Yi MC, et al. Bioorg Med Chem Lett. 2015 Nov 1;25(21):4922-4926. doi: 10.1016/j.bmcl.2015.05.006. Epub 2015 May 15. Bioorg Med Chem Lett. 2015. PMID: 26004580 Free PMC article. - Dihydroisoxazole analogs for labeling and visualization of catalytically active transglutaminase 2.
Dafik L, Khosla C. Dafik L, et al. Chem Biol. 2011 Jan 28;18(1):58-66. doi: 10.1016/j.chembiol.2010.11.004. Chem Biol. 2011. PMID: 21276939 Free PMC article. - Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles.
Watts RE, Siegel M, Khosla C. Watts RE, et al. J Med Chem. 2006 Dec 14;49(25):7493-501. doi: 10.1021/jm060839a. J Med Chem. 2006. PMID: 17149878 Free PMC article. - Transglutaminase 2 inhibitors and their therapeutic role in disease states.
Siegel M, Khosla C. Siegel M, et al. Pharmacol Ther. 2007 Aug;115(2):232-45. doi: 10.1016/j.pharmthera.2007.05.003. Epub 2007 May 13. Pharmacol Ther. 2007. PMID: 17582505 Free PMC article. Review. - Role of transglutaminase 2 in celiac disease pathogenesis.
Klöck C, Diraimondo TR, Khosla C. Klöck C, et al. Semin Immunopathol. 2012 Jul;34(4):513-22. doi: 10.1007/s00281-012-0305-0. Epub 2012 Mar 22. Semin Immunopathol. 2012. PMID: 22437759 Free PMC article. Review.
Cited by
- Design, synthesis and biological evaluation of novel indolin-2-ones as potent anticancer compounds.
Zhou A, Yan L, Lai F, Chen X, Goto M, Lee KH, Xiao Z. Zhou A, et al. Bioorg Med Chem Lett. 2017 Aug 1;27(15):3326-3331. doi: 10.1016/j.bmcl.2017.06.019. Epub 2017 Jun 7. Bioorg Med Chem Lett. 2017. PMID: 28625363 Free PMC article. - Inhibition of hypoxia-inducible factor via upregulation of von Hippel-Lindau protein induces "angiogenic switch off" in a hepatoma mouse model.
Iwamoto H, Nakamura T, Koga H, Izaguirre-Carbonell J, Kamisuki S, Sugawara F, Abe M, Iwabata K, Ikezono Y, Sakaue T, Masuda A, Yano H, Ohta K, Nakano M, Shimose S, Shirono T, Torimura T. Iwamoto H, et al. Mol Ther Oncolytics. 2015 Dec 2;2:15020. doi: 10.1038/mto.2015.20. eCollection 2015. Mol Ther Oncolytics. 2015. PMID: 27119112 Free PMC article. - Cystamine and Disulfiram Inhibit Human Transglutaminase 2 via an Oxidative Mechanism.
Palanski BA, Khosla C. Palanski BA, et al. Biochemistry. 2018 Jun 19;57(24):3359-3363. doi: 10.1021/acs.biochem.8b00204. Epub 2018 Mar 28. Biochemistry. 2018. PMID: 29570977 Free PMC article. - Synthesis and evaluation of oxindoles as promising inhibitors of the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1.
Paul S, Roy A, Deka SJ, Panda S, Srivastava GN, Trivedi V, Manna D. Paul S, et al. Medchemcomm. 2017 Jun 20;8(8):1640-1654. doi: 10.1039/c7md00226b. eCollection 2017 Aug 1. Medchemcomm. 2017. PMID: 30108875 Free PMC article. - Chemical Constituents of Phaius mishmensis.
Jao CW, Hung TH, Chang CF, Chuang TH. Jao CW, et al. Molecules. 2016 Nov 23;21(11):1605. doi: 10.3390/molecules21111605. Molecules. 2016. PMID: 27886100 Free PMC article.
References
- Fesus L, Piacentini M. Trends Biochem. Sci. 2002;27:534–539. - PubMed
- Bailey CDC, Tucholski J, Johnson GVW. Progress in experimental tumor research. 2005;38:139–157. - PubMed
- Sollid LM. Nat Rev Immunol. 2002;2:647–655. - PubMed
- Ruan Q, Johnson GVW. Front. Biosci. 2007;3:891–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information