Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes - PubMed (original) (raw)
Review
Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes
Dylan Dodd et al. Mol Microbiol. 2011 Jan.
Abstract
Microbial inhabitants of the bovine rumen fulfil the majority of the normal caloric requirements of the animal by fermenting lignocellulosic plant polysaccharides and releasing short chain fatty acids that are then metabolized by the host. This process also occurs within the human colon, although the fermentation products contribute less to the overall energy requirements of the host. Mounting evidence, however, indicates that the community structure of the distal gut microbiota is a critical factor that influences the inflammatory potential of the immune system thereby impacting the progression of inflammatory bowel diseases. Non-digestible dietary fibre derived from plant material is highly enriched in the lignocellulosic polysaccharides, cellulose and xylan. Members of the Bacteroidetes constitute a dominant phylum in both the human colonic microbiome and the rumen microbial ecosystem. In the current article, we review recent insights into the molecular mechanisms for xylan degradation by rumen and human commensal members of the Bacteroidetes phylum, and place this information in the context of the physiological and metabolic processes that occur within these complex microbial environments.
© 2010 Blackwell Publishing Ltd.
Figures
Fig. 1
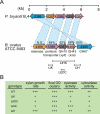
A. Conservation of xylan hydrolase gene clusters in B. ovatus ATCC 8483 and P. bryantii B14. The corresponding genes in the two bacteria are connected by dashed lines, and the per cent amino acid identity between the gene products is indicated. Open reading frame numbers are indicated within each of the genes. N.S., not significant amino acid identity. A map of insertional mutants constructed in the chromosome of B. ovatus ATCC 8483, as described in the text, is indicated below the corresponding genes. B. Phenotypes associated with the insertional mutants indicated in (A) and described in the text. Data are derived from Weaver et al. (1992). OD, optical density.
Fig. 2
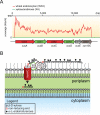
Model for the xylan utilization system in xylanolytic Bacteroidetes. A. RNAseq coverage map of the major xylan utilization system in P. bryantii B14 during growth on soluble wheat arabinoxylan (WAX, red line) or a mixture of xylose and arabinose (XA, green line). Total RNA was extracted from P. bryantii B14 cultured on either growth substrate (WAX or XA), and then rRNA was subtracted and the enriched mRNA was converted to cDNA. The cDNA was directly sequenced by Illumina technology and individual sequence reads were assembled onto the genome sequence of P. bryantii B14. These data are derived from Dodd et al. (2010a). B. Predicted model for binding of xylan, cleavage and transport of xylan fragments across the outer membrane by components of the Xus cluster in P. bryantii B14. The proteins, XusA and XusC, are homologues of the SusC TonB-dependent receptor, which is involved in oligosaccharide transport across the outer membrane in B. thetaiotaomicron VPI-5482. XusB and XusD are homologues of B. thetaiotaomicron VPI-5482 SusD, which binds polysaccharides on the outer leaflet of the outer membrane. XusE has no homology to other characterized proteins, but the presence of an N-terminal signal peptidase II cleavage site in this protein suggests that it is tethered to the outer leaflet of the outermembrane. Xyn10C is an endoxylanase gene that also possesses a putative N-terminal signal peptidase cleavage II site. In the predicted model, XusE, Xyn10C and the XusB/D proteins bind to extracellular xylan polymers. Xyn10C then catalyses the endo-cleavage of these polymers and XusB/D facilitate transport of these fragments across the outer membrane and into the periplasm through the TonB-dependent receptors XusA and XusC.
Fig. 3
Presence of carbohydrate active enzymes associated with xylan degradation in Bacteroidetes genomes that possess a Xus gene cluster. A. The general structure for xylan is shown and the relevant glycoside hydrolase (GH) enzymes are indicated. Only the GH families that are present within the genome sequences of Xus-containing Bacteroidetes are shown. B. Frequency distribution plot for the GH families from panel A in xylanolytic Bacteroidetes. For GH 5, only those members with high homology [expect (E) value < 1 × 10−15] to the Bacteroidetes-specific endoxylanase enzymes identified by Dodd et al. (2010a) are included. With the exception of Prevotella ruminicola 23, which has a complete genome, all numbers of genes are estimates based on the analysis of data from partially sequenced genomes. Analyses were performed by identifying a biochemically verified member from each GH family on the CAZy website, and using it as a query for a BLASTp search of the listed bacterial genome. Only those results that exhibited _E_-values < 1 × 10−5 were included.
Fig. 4
Expansion of xylanase enzymes in the genome of Bacteroides intestinalis DSM 17393. Putative GH family 10 xylanase enzymes were identified in the B. intestinalis genome using P. bryantii B14 Xyn10A (Gasparic et al., 1995b) as a query in a BLASTp search of the genome. Domain architectures were predicted using the Conserved Domains Database (CDD) on the NCBI website (Marchler-Bauer et al., 2007). Domains were included if the _E_-value was less than 1 × 10−5. The two biochemically verified (Dodd et al., 2010a) GH 5 endoxylanases from B. intestinalis DSM 17393 are also included and indicated by asterisks (BACINT_01125 and BACINT_04213). Signal peptides and lipoprotein signal sequences were predicted using SignalP v3.0 (Emanuelsson et al., 2007) and LipoP v1.0 (Juncker et al., 2003) respectively. N.S., no significant match to any domain within the CD database.
Fig. 5
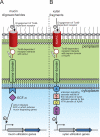
Model for signal transduction in gut Bacteroidetes. A. Mechanism revealed by Martens et al., (2009b) for _trans_-envelope signalling in response to mucin oligosaccharide degradation by Bacteroides thetaiotaomicron VPI-5482. Mucin oligosaccharides engage a TonB-dependent receptor, which releases the N-terminal plug domain of the receptor. The N-terminal extension of the TonB receptor is then able to interact with the sensor domain of an anti-σ factor. This interaction causes the anti-σ to release the ECF-σ that was being sequestered in the cytoplasm and is now free to induce the transcription of mucin utilization genes. B. Potential model for xylan signal recognition involving XusC/D and XynR. Extracellular xylan fragments engage the TonB-dependent receptors XusC and/or XusD and this triggers release of the N-terminal plug domain of the receptor. The N-terminal extension of XusC/D is then able to interact with the sensor domain of XynR. An unknown signalling cascade, probably involving the histidine kinase (H and HK) and response regulator (D and HTH) domains, activates the C-terminal helix-turn-helix (HTH) domain of XynR, allowing it to bind to regulatory elements and thus inducing transcription of xylan utilization genes.
Fig. 6
Genomic organization of xylulokinase genes from selected human or bovine-associated Bacteroidetes. The Prevotella bryantii B14 xylulokinase gene (GenBank accession no. EFI71375.1) was used as the query sequence in a BLASTp search of the GenBank database. Next, the Bacteroides thetaiotaomicron VPI-5482 xylose isomerase gene (BT_0793) was used as a query sequence in a BLASTp search of the GenBank database. The four organisms that possess only a xylulose kinase gene and no xylose isomerase gene are grouped in the upper half of the figure, and representative organisms that contain both genes are grouped in the lower half. The genomic context is shown for the region surrounding the xylulose kinase genes in these organisms. ORF numbers are indicated within each of the genes as derived from the genome project for each organism in the GenBank database. The small subunit (16S) ribosomal RNA genes from the organisms were aligned and a neighbour-joining tree was constructed using the CLC Genomics Workbench v3.0 software. Each alignment was re-sampled 100 times and the bootstrap values are indicated on the internal branches. The branch length is reported as the expected number of substitutions per nucleotide position. The rumen isolates are in pink and the human isolates are colour-coded as indicated in the legend based upon the reported body isolation site as listed in the Human Microbiome Projects Catalog (
).
Similar articles
- New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11.
Solden LM, Hoyt DW, Collins WB, Plank JE, Daly RA, Hildebrand E, Beavers TJ, Wolfe R, Nicora CD, Purvine SO, Carstensen M, Lipton MS, Spalinger DE, Firkins JL, Wolfe BA, Wrighton KC. Solden LM, et al. ISME J. 2017 Mar;11(3):691-703. doi: 10.1038/ismej.2016.150. Epub 2016 Dec 13. ISME J. 2017. PMID: 27959345 Free PMC article. - Transcriptomic analyses of xylan degradation by Prevotella bryantii and insights into energy acquisition by xylanolytic bacteroidetes.
Dodd D, Moon YH, Swaminathan K, Mackie RI, Cann IK. Dodd D, et al. J Biol Chem. 2010 Sep 24;285(39):30261-73. doi: 10.1074/jbc.M110.141788. Epub 2010 Jul 9. J Biol Chem. 2010. PMID: 20622018 Free PMC article. - Shifts in xylanases and the microbial community associated with xylan biodegradation during treatment with rumen fluid.
Takizawa S, Asano R, Fukuda Y, Baba Y, Tada C, Nakai Y. Takizawa S, et al. Microb Biotechnol. 2022 Jun;15(6):1729-1743. doi: 10.1111/1751-7915.13988. Epub 2021 Dec 28. Microb Biotechnol. 2022. PMID: 34964273 Free PMC article. Review. - Do rumen Bacteroidetes utilize an alternative mechanism for cellulose degradation?
Naas AE, Mackenzie AK, Mravec J, Schückel J, Willats WG, Eijsink VG, Pope PB. Naas AE, et al. mBio. 2014 Aug 5;5(4):e01401-14. doi: 10.1128/mBio.01401-14. mBio. 2014. PMID: 25096880 Free PMC article. - Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses.
Gharechahi J, Vahidi MF, Sharifi G, Ariaeenejad S, Ding XZ, Han JL, Salekdeh GH. Gharechahi J, et al. Environ Res. 2023 Jul 15;229:115925. doi: 10.1016/j.envres.2023.115925. Epub 2023 Apr 21. Environ Res. 2023. PMID: 37086884 Review.
Cited by
- Inclusion of chicory (Cichorium intybus L.) in pigs' diets affects the intestinal microenvironment and the gut microbiota.
Liu H, Ivarsson E, Dicksved J, Lundh T, Lindberg JE. Liu H, et al. Appl Environ Microbiol. 2012 Jun;78(12):4102-9. doi: 10.1128/AEM.07702-11. Epub 2012 Apr 6. Appl Environ Microbiol. 2012. PMID: 22492453 Free PMC article. - Description of a novel pectin-degrading bacterial species Prevotella pectinovora sp. nov., based on its phenotypic and genomic traits.
Nograšek B, Accetto T, Fanedl L, Avguštin G. Nograšek B, et al. J Microbiol. 2015 Aug;53(8):503-10. doi: 10.1007/s12275-015-5142-0. Epub 2015 Jul 31. J Microbiol. 2015. PMID: 26224452 - Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on the Qinghai-Tibetan plateau.
Liu H, Xu T, Xu S, Ma L, Han X, Wang X, Zhang X, Hu L, Zhao N, Chen Y, Pi L, Zhao X. Liu H, et al. PeerJ. 2019 Aug 5;7:e7462. doi: 10.7717/peerj.7462. eCollection 2019. PeerJ. 2019. PMID: 31404417 Free PMC article. - New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11.
Solden LM, Hoyt DW, Collins WB, Plank JE, Daly RA, Hildebrand E, Beavers TJ, Wolfe R, Nicora CD, Purvine SO, Carstensen M, Lipton MS, Spalinger DE, Firkins JL, Wolfe BA, Wrighton KC. Solden LM, et al. ISME J. 2017 Mar;11(3):691-703. doi: 10.1038/ismej.2016.150. Epub 2016 Dec 13. ISME J. 2017. PMID: 27959345 Free PMC article. - Cover crop monocultures and mixtures enhance bacterial abundance and functionality in the maize root zone.
Ghosh D, Shi Y, Zimmermann IM, Stürzebecher T, Holzhauser K, von Bergen M, Kaster AK, Spielvogel S, Dippold MA, Müller JA, Jehmlich N. Ghosh D, et al. ISME Commun. 2024 Oct 29;4(1):ycae132. doi: 10.1093/ismeco/ycae132. eCollection 2024 Jan. ISME Commun. 2024. PMID: 39526131 Free PMC article.
References
- Avgustin G, Wallace RJ, Flint HJ. Phenotypic diversity among ruminal isolates of Prevotella ruminicola: proposal of Prevotella brevis sp. nov., Prevotella bryantii sp. nov., and Prevotella albensis sp. nov. and redefinition of Prevotella ruminicola. Int J Syst Bacteriol. 1997;47:284–288. - PubMed
- Bakir MA, Kitahara M, Sakamoto M, Matsumoto M, Benno Y. Bacteroides intestinalis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2006;56:151–154. - PubMed
- Bjursell MK, Martens EC, Gordon JI. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J Biol Chem. 2006;281:36269–36279. - PubMed
- Braun V, Mahren S. Transmembrane transcriptional control (surface signalling) of the Escherichia coli. Fec type. FEMS Microbiol Rev. 2005;29:673–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases