Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents - PubMed (original) (raw)
Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents
Alec Okun et al. Mol Pain. 2011.
Abstract
Background: Tissue injury elicits both hypersensitivity to evoked stimuli and ongoing, stimulus-independent pain. We previously demonstrated that pain relief elicits reward in nerve-injured rats. This approach was used to evaluate the temporal and mechanistic features of inflammation-induced ongoing pain.
Results: Intraplantar Complete Freund's Adjuvant (CFA) produced thermal hyperalgesia and guarding behavior that was reliably observed within 24 hrs and maintained, albeit diminished, 4 days post-administration. Spinal clonidine produced robust conditioned place preference (CPP) in CFA treated rats 1 day, but not 4 days following CFA administration. However, spinal clonidine blocked CFA-induced thermal hyperalgesia at both post-CFA days 1 and 4, indicating different time-courses of ongoing and evoked pain. Peripheral nerve block by lidocaine administration into the popliteal fossa 1 day following intraplantar CFA produced a robust preference for the lidocaine paired chamber, indicating that injury-induced ongoing pain is driven by afferent fibers innervating the site of injury. Pretreatment with resiniferatoxin (RTX), an ultrapotent capsaicin analogue known to produce long-lasting desensitization of TRPV1 positive afferents, fully blocked CFA-induced thermal hypersensitivity and abolished the CPP elicited by administration of popliteal fossa lidocaine 24 hrs post-CFA. In addition, RTX pretreatment blocked guarding behavior observed 1 day following intraplantar CFA. In contrast, administration of the selective TRPV1 receptor antagonist, AMG9810, at a dose that reversed CFA-induced thermal hyperalgesia failed to reduce CFA-induced ongoing pain or guarding behavior.
Conclusions: These data demonstrate that inflammation induces both ongoing pain and evoked hypersensitivity that can be differentiated on the basis of time course. Ongoing pain (a) is transient, (b) driven by peripheral input resulting from the injury, (c) dependent on TRPV1 positive fibers and (d) not blocked by TRPV1 receptor antagonism. Mechanisms underlying excitation of these afferent fibers in the early post-injury period will offer insights for development of novel pain relieving strategies in the early post-traumatic period.
Figures
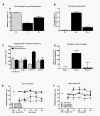
Figure 1
A. Hindpaw CFA produced lower paw withdrawal latencies within 24 hours (D1). Paw withdrawal latencies remained lower than pre-injury baseline (BL) 4 days following injury, but were higher than those observed 1 day following injury. *p < 0.05 vs. pre-injury thresholds (BL); #p < 0.05 vs. D1 thresholds, n = 7-8. B. Hindpaw CFA produced guarding behavior within 24 hours (D1). Guarding behavior remained elevated compared to pre-injury baselines (BL) 4 days following injury, but was significantly reduced compared to the D1 time-point. *p < 0.05 vs. pre-injury thresholds (BL); #p < 0.05 vs. D1 thresholds, n = 7-8. C. Spinal clonidine (10 μg) induced CPP selectively in CFA treated rats at day 1, but not day 4 post CFA, injection. No chamber preference is observed in saline treated rats indicating that this dose of spinal clonidine is not rewarding in the absence of injury. *p < 0.05 vs. saline paired chamber; #p < 0.05 vs. pre-conditioning, n = 7. D. Difference scores calculated as test time - preconditioning time spent in clonidine chamber confirm that CFA treated rats showed CPP 24 hrs, but not 4 days post CFA injection. *p < 0.05 vs. saline treated group. E. CFA decreased paw withdrawal latencies to radiant heat within 24 hours of injection (Post-CFA). Spinal clonidine (10 μg) attenuated, but did not fully reverse CFA-induced thermal hyperalgesia. This dose of spinal clonidine failed to induce antinociception in rats that received intraplantar saline, *p < 0.05 vs. post-CFA, #p < 0.05 vs. pre-CFA, n = 5-8. F. CFA-induced thermal hyperalgesia persisted through day 4 post-CFA. Spinal clonidine (10 μg) induced antihyperalgesia and antinociception, elevating paw withdrawal latencies above baseline values. *p < 0.05 vs. post-CFA, #p < 0.05 vs. pre-CFA, n = 5-8.
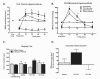
Figure 2
A. CFA reduced withdrawal latency within 24 hrs post-CFA. Popliteal fossa lidocaine (4% w/v, 200 μl) reversed CFA-induced hypersensitivity, elevating paw withdrawal latencies to noxious thermal stimulation to almost cut-off in both CFA and control (saline) treated rats at the 15 min time-point. Elevated paw-flick latencies were observed throughout the 60 min testing period in the CFA treated rats, and returned to pre-lidocaine latencies within 45 min in the control rats, *p < 0.05 vs. post-CFA, #p < 0.05 vs. pre-CFA, n = 5-6. B. CFA decreased withdrawal thresholds to noxious mechanical stimulation within 24 hrs. Popliteal fossa lidocaine elevated paw-withdrawal thresholds to almost cut-off in both the CFA and saline (control) groups at the 15 min time-point. Paw-withdrawal thresholds returned to pre-lidocaine values within 30 min for control rats, and within 45 min in the CFA treated rats, *p < 0.05 vs. post-CFA, #p < 0.05 vs. pre-CFA, n = 5-6. C. Popliteal fossa lidocaine induced CPP 24 hrs, but not 4-days post-CFA injection. *p < 0.05 vs. pre-conditioning, p < 0.05, n = 5-6. D. Difference scores calculated as test time - preconditioning time spent in lidocaine chamber confirm that only rats treated with CFA 24 hours prior to conditioning showed CPP in response to peripheral nerve block by lidocaine. *p < 0.05 vs. saline treated group.
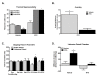
Figure 3
A. RTX (0.1 mg/kg, i.p.) treatment 4 days prior to testing blocked thermal nociception with all responses on the hot plate (52°C) reaching the cut-off time (32 s). CFA reduced the thermal response latency within 24 hr in animals that received vehicle for RTX. Thermal hypersensitivity was completely blocked in the RTX treated group. *p < 0.05 vs. pre-RTX; #p < 0.05 vs. vehicle, n = 7. B. RTX treatment 4 days prior to testing blocked CFA induced guarding of the hindpaw observed 24 hours following injury. *p < 0.05 vs. vehicle treated, n = 8-10. C. RTX treatment 1 day prior to testing prior to habituation blocked CPP to chambers paired with lidocaine administration into the popliteal fossa in CFA treated rats 24 hr post-CFA. Rats that received saline into the hindpaw failed to show preference for the lidocaine paired chamber irrespective of RTX treatment. *p < 0.05 vs. pre-conditioning, n = 6-8. D. Difference scores calculated as test time - preconditioning time spent in lidocaine chamber confirm that only CFA treated rats that did not receive RTX (Vehicle) showed preference for the lidocaine paired chamber. *p < 0.05 vs. saline treated group.
Figure 4
A. CFA reduced withdrawal latency within 24 hrs post-CFA. AMG9810 (30 mg/kg, i.p.) reversed CFA-induced thermal hyperalgesia within 15 min, with paw-flick latencies returning to pre-drug levels 60 min post administration. AMG9810 also elevated paw-flick latencies of control (saline treated) rats with 15 min of administration. *p < 0.05 vs. post-CFA, #p < 0.05 vs. pre-CFA, n = 5-6. B. Systemic administration of the selective TRPV1 receptor antagonist, AMG9810 (30 mg/kg i.p.) failed to alter CFA-induced guarding behavior 24 hours following injury, p > 0.05 vs. vehicle. C. Pre-conditioning values did not differ between treatment groups, therefore values were pooled for graphical representation. Lidocaine increased time spent in the lidocaine paired chambers in both the AMG9810 and vehicle treated rats, *p < 0.05 vs. pre-conditioning, n = 5. D. Difference scores calculated as test time - preconditioning time spent in the lidocaine chamber confirm that CFA treated rats increased time spent in the lidocaine paired chambers in both the vehicle and AMG9810 treatment groups, *p < 0.05 vs. saline/vehicle.
Similar articles
- Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia.
Kanai Y, Hara T, Imai A, Sakakibara A. Kanai Y, et al. J Pharm Pharmacol. 2007 May;59(5):733-8. doi: 10.1211/jpp.59.5.0015. J Pharm Pharmacol. 2007. PMID: 17524240 - The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund's adjuvant.
Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, Wan Y. Yu L, et al. Mol Pain. 2008 Dec 4;4:61. doi: 10.1186/1744-8069-4-61. Mol Pain. 2008. PMID: 19055783 Free PMC article. - RNA interference-mediated knock-down of transient receptor potential vanilloid 1 prevents forepaw inflammatory hyperalgesia in rat.
Kasama S, Kawakubo M, Suzuki T, Nishizawa T, Ishida A, Nakayama J. Kasama S, et al. Eur J Neurosci. 2007 May;25(10):2956-63. doi: 10.1111/j.1460-9568.2007.05584.x. Epub 2007 May 17. Eur J Neurosci. 2007. PMID: 17509082 - Possible consequences of blocking transient receptor potential vanilloid.
Bishnoi M, Premkumar LS. Bishnoi M, et al. Curr Pharm Biotechnol. 2011 Jan 1;12(1):102-14. doi: 10.2174/138920111793937907. Curr Pharm Biotechnol. 2011. PMID: 20932252 Review. - Resiniferatoxin: Nature's Precision Medicine to Silence TRPV1-Positive Afferents.
Szallasi A. Szallasi A. Int J Mol Sci. 2023 Oct 10;24(20):15042. doi: 10.3390/ijms242015042. Int J Mol Sci. 2023. PMID: 37894723 Free PMC article. Review.
Cited by
- TIMP-1 Attenuates the Development of Inflammatory Pain Through MMP-Dependent and Receptor-Mediated Cell Signaling Mechanisms.
Knight BE, Kozlowski N, Havelin J, King T, Crocker SJ, Young EE, Baumbauer KM. Knight BE, et al. Front Mol Neurosci. 2019 Sep 20;12:220. doi: 10.3389/fnmol.2019.00220. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31616247 Free PMC article. - Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives.
Shiao R, Lee-Kubli CA. Shiao R, et al. Neurotherapeutics. 2018 Jul;15(3):635-653. doi: 10.1007/s13311-018-0633-4. Neurotherapeutics. 2018. PMID: 29736857 Free PMC article. Review. - Reward and motivation in pain and pain relief.
Navratilova E, Porreca F. Navratilova E, et al. Nat Neurosci. 2014 Oct;17(10):1304-12. doi: 10.1038/nn.3811. Epub 2014 Sep 25. Nat Neurosci. 2014. PMID: 25254980 Free PMC article. Review. - The application of conditioning paradigms in the measurement of pain.
Li JX. Li JX. Eur J Pharmacol. 2013 Sep 15;716(1-3):158-68. doi: 10.1016/j.ejphar.2013.03.002. Epub 2013 Mar 13. Eur J Pharmacol. 2013. PMID: 23500202 Free PMC article. Review.
References
- Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical