Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans - PubMed (original) (raw)
Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans
Boris Calderon et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Understanding the entry of autoreactive T cells to their target organ is important in autoimmunity because this entry initiates the inflammatory process. Here, the events that lead to specific localization of diabetogenic CD4 T cells into islets of Langerhans resulting in diabetes were examined. This was evaluated in two models, one in which T cells specific for a hen-egg white lysozyme (HEL) peptide were injected into mice expressing HEL on β cells and the other using T cells in the nonobese diabetic mouse strain, which develops spontaneous diabetes. Only T cells specific for β-cell antigens localized in islets within the first hours after their injection and were found adherent to intraislet dendritic cells (DCs). DCs surrounded blood vessels with dendrites reaching into the vessels. Localization of antigen-specific T cells did not require chemokine receptor signaling but involved class II histocompatibility and intercellular adhesion molecule 1 molecules. We found no evidence for nonspecific localization of CD4 T cells into normal noninflamed islets. Thus, the anatomy of the islet of Langerhans permits the specific localization of diabetogenic T cells at a time when there is no inflammation in the islets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
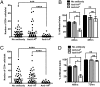
Fig. 1.
Localization of CD4 T cells to islets containing their antigen and MHC class II requirement. (A) Pooled results showing localization of activated 3A9 T cells into sublethally irradiated B10.BR and IP-HEL recipients at different times. Numbers of experiments are indicated on each bar. (B) Transfer of activated or unstimulated BDC T cells into NOD _Rag1_−/− recipients, analyzed at different time points. Numbers of experiments are indicated on each bar. (C) Localization of CD4 T cells into MHC class II-sufficient or -deficient mice. Activated BDC T cells were transferred into sublethally irradiated 8-wk-old NOD male mice (MHC II+/+) or MHC class II−/− deficient mice. The graph shows the number of infiltrated CD4 T cells per islet at 48 h after cell transfer. (D) Percentage of infiltrated islets from previous experiments (C). Data are represented as the mean (±SEM).
Fig. 2.
Localization of CD4 T cells to islets during partial inhibition by anti-class II MHC mAb. (A) T-cell distribution in islets 24 h after transfer of activated 3A9 T cells into IP-HEL mice treated as indicated, 1 d before cell transfer. The graph shows a representative experiment from a total of six, and each dot represents an islet. (B) Percentage of T cell-infiltrated islets at 24 and 48 h in IP-HEL mice treated as in A. Results are pooled from six and four experiments at 24 and 48 h, respectively. (C) T-cell distribution in islets 48 h after transfer of activated BDC T cells into NOD _Rag1_−/− mice treated as indicated. The graph shows a representative experiment from four experiments. (D) Percentage of T cell-infiltrated islets at 48 and 72 h in mice treated as in C. Results are pooled from four experiments. Data are represented as the mean (±SEM). P ≥ 0.05 [not significant (ns)]; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. 3.
Partial inhibition of T-cell localization by anti-class II MHC and ICAM-1 mAb. (A) T-cell distribution in islets 24 h after transfer of activated 3A9 T cells into IP-HEL mice treated with a single mAb or a combination of both, 1 d before cell transfer. The graph shows a representative experiment from a total of two experiments. (B) Percentage of infiltrated islets from the previous experiment (A), showing the compiled data from two experiments. (C) T-cell distribution in islets 48 h after the transfer of activated BDC T cells into sublethally irradiated NOD.ICAM-1+/+ and NOD.ICAM-1−/− mice treated with a single anti-class II blocking mAb as indicated, 1 d before cell transfer. The graph shows a representative experiment from a total of two experiments. (D) Percentage of infiltrated islets from the previous experiment (C), showing the compiled data from two experiments at 48 h. Data are represented as the mean (±SEM). P ≥ 0.05 [not significant (ns)]; *P < 0.05; ***P < 0.001; ****P < 0.0001.
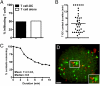
Fig. 4.
T cell-DC interaction in islets by two-photon microscopy and immunofluorescence. (A) Live isolated islet analysis evaluating percentage of 3A9 T cells found in contact with islet DCs at 24 h after cell transfer. T cell-DC contacts were obtained from a total of 20 examined islets from two independent experiments. 3A9 T cells were detected with anti-CD4 mAb, and DCs were detected with anti-CD11c mAb. (B) Plot shows T cell-DC contact durations of 3A9 (CFSE-labeled) T cells at 24 h after cell transfer in IP-HEL mice. Contact durations were obtained from 10- to 15-min time-lapse movies from two experiments. (C) Analysis showing percentage of T cell-DC remaining contact for any given duration. (D) Infiltrated islet at 24 h after activated T-cell transfer showing 3A9 T cells (red) and CD11c cells (green). (Inset) Micrograph showing contact of T cells with CD11c cells (yellow merged color). (Scale bar, 50 μm.)
Fig. 5.
Analysis of islet DCs in direct contact with blood vessels. (A) Two-photon microscopic analysis of a representative islet of C57BL/6 mice expressing YFP under the CD11c promoter (CD11c-YFP, shown in green) and endothelia stained with PECAM-1 (red). Reconstruction from a single stack (1-μm thickness). The arrow shows a DC in apposition to the vessel and dendrite, reaching into the lumen (yellow merging color). (B) 3D reconstruction by confocal microscopy of an islet from CD11c-YFP mice with vessels stained in red (PECAM-1) and CD11c-YFP (green). (Lower Left) Projection along the z axis (top view) from a stack of 30 optical sections (0.5-μm increments). (Upper Right) z–x and z–y reconstructions (side view) of same image stack (indicated as white lines). The image shows a DC dendrite inside the vessel (yellow merged color indicated by arrows). (C) Percentages of DCs contacting the vessel wall (not embedded), dendrites in the vessel wall, or DCs protruding into the lumen (n = 108). Data were obtained from 17 different visualized fields from two experiments. (D) Quantitative analysis of anti-class II mAb fluorescent bead (anti–I-Ak or anti–I-Ag7) localization. Each dot represents a single islet. The left and right y axes show the number of anti-class II beads per islet in B10.BR and NOD mice, respectively, representing compiled data from three independent experiments. **P < 0.01; ****P < 0.0001. (E) Localization of anti-class II–coated beads by immunofluorescence in islets of B10.BR mice previously injected i.v. with beads coated with anti–I-Ak. Islet DCs are shown in green (CD11c), blood vessels are shown in blue (PECAM-1), and fluorescent beads are shown in red. (Insets) Micrograph showing a representative islet DC in contact with a bead inside the lumen of the vessel. Bead and PECAM-1 (Bottom Right); CD11c and PECAM-1 (Middle Right); bead, CD11c, and PECAM-1 (Top Right). (Scale bar, 20 μm.)
Similar articles
- Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - tRNA-derived fragments in T lymphocyte-beta cell crosstalk and in type 1 diabetes pathogenesis in NOD mice.
Brozzi F, Jacovetti C, Cosentino C, Menoud V, Wu K, Bayazit MB, Abdulkarim B, Iseli C, Guex N, Guay C, Regazzi R. Brozzi F, et al. Diabetologia. 2024 Oct;67(10):2260-2274. doi: 10.1007/s00125-024-06207-3. Epub 2024 Jul 5. Diabetologia. 2024. PMID: 38967669 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Conservative, physical and surgical interventions for managing faecal incontinence and constipation in adults with central neurological diseases.
Todd CL, Johnson EE, Stewart F, Wallace SA, Bryant A, Woodward S, Norton C. Todd CL, et al. Cochrane Database Syst Rev. 2024 Oct 29;10(10):CD002115. doi: 10.1002/14651858.CD002115.pub6. Cochrane Database Syst Rev. 2024. PMID: 39470206
Cited by
- Macrophages in diabetes mellitus (DM) and COVID-19: do they trigger DM?
Kloc M, Ghobrial RM, Lewicki S, Kubiak JZ. Kloc M, et al. J Diabetes Metab Disord. 2020 Oct 17;19(2):2045-2048. doi: 10.1007/s40200-020-00665-3. eCollection 2020 Dec. J Diabetes Metab Disord. 2020. PMID: 33102261 Free PMC article. - Coreceptor therapy has distinct short- and long-term tolerogenic effects intrinsic to autoreactive effector T cells.
Clark M, Kroger CJ, Ke Q, Zhang R, Statum K, Milner JJ, Martin A, Wang B, Tisch R. Clark M, et al. JCI Insight. 2021 Sep 8;6(17):e149130. doi: 10.1172/jci.insight.149130. JCI Insight. 2021. PMID: 34314385 Free PMC article. - Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse.
Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Carrero JA, et al. PLoS One. 2013;8(3):e59701. doi: 10.1371/journal.pone.0059701. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555752 Free PMC article. - Beta cell antigens in type 1 diabetes: triggers in pathogenesis and therapeutic targets.
Mauvais FX, Diana J, van Endert P. Mauvais FX, et al. F1000Res. 2016 Apr 22;5:F1000 Faculty Rev-728. doi: 10.12688/f1000research.7411.1. eCollection 2016. F1000Res. 2016. PMID: 27158463 Free PMC article. Review. - Population dynamics of islet-infiltrating cells in autoimmune diabetes.
Magnuson AM, Thurber GM, Kohler RH, Weissleder R, Mathis D, Benoist C. Magnuson AM, et al. Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1511-6. doi: 10.1073/pnas.1423769112. Epub 2015 Jan 20. Proc Natl Acad Sci U S A. 2015. PMID: 25605891 Free PMC article.
References
- Gallatin M, et al. Lymphocyte homing receptors. Cell. 1986;44:673–680. - PubMed
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. - PubMed
- Krishnamoorthy G, Wekerle H. EAE: An immunologist's magic eye. Eur J Immunol. 2009;39:2031–2035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK058177/DK/NIDDK NIH HHS/United States
- AI024742/AI/NIAID NIH HHS/United States
- R01 AI024742/AI/NIAID NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P60DK20579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials