Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens - PubMed (original) (raw)
Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens
Daniela Jabés et al. Antimicrob Agents Chemother. 2011 Apr.
Abstract
NAI-107 is a novel lantibiotic active against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), glycopeptide-intermediate S. aureus (GISA), and vancomycin-resistant enterococci (VRE). The aim of this study was to evaluate the in vivo efficacy of NAI-107 in animal models of severe infection. In acute lethal infections induced with a penicillin-intermediate Streptococcus pneumoniae strain in immunocompetent mice, or with MRSA, GISA, and VRE strains in neutropenic mice, the 50% effective dose (ED(50)) values of NAI-107 were comparable or lower than those of reference compounds, irrespective of the strain and immune status (0.51 to 14.2 mg/kg of body weight for intravenous [i.v.] NAI-107, 5.1 to 22.4 for oral linezolid, and 22.4 for subcutaneous [s.c.] vancomycin). In the granuloma pouch model induced in rats with a MRSA strain, intravenous NAI-107 showed a dose-proportional bactericidal activity that, at a single 40-mg/kg dose, compared with 2 20-mg/kg doses at a 12-h or 24-h interval, caused a 3-log(10)-CFU/ml reduction of viable MRSA in exudates that persisted for more than 72 h. Rat endocarditis was induced with a MRSA strain and treated for five consecutive days. In a first experiment, using 5, 10, or 20 mg/kg/day, and in a second experiment, when 10 mg/kg at 12-h intervals was compared to 20 mg/kg/day, intravenous NAI-107 was effective in reducing the bacterial load in heart vegetations in a dose-proportional manner. Trough plasma levels, as determined on days 2 and 5, were several times higher than the NAI-107 minimal bactericidal concentration (MBC). NAI-107 binding to rat and human serum ranges between 93% and 98.6%. The rapid bactericidal activity of NAI-107 observed in vitro was thus confirmed by the efficacy in several models of experimental infection induced by Gram-positive pathogens, supporting further investigation of the compound.
Figures
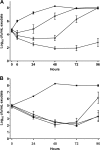
FIG. 1.
In vivo bactericidal activity of NAI-107 in the rat granuloma pouch model induced by S. aureus 1400. (A) Effects of single 10-mg/kg (circles), 20-mg/kg (triangles), and 40-mg/kg (diamonds) i.v. doses on bacterial counts (log10 CFU/ml) in pouch exudates over time. (B) Effects of 2 20-mg/kg doses i.v. every 24 h (q24) (filled triangles) and 2 20-mg/kg doses i.v. q12 (empty triangle) in comparison with two 100-mg/kg doses i.m. q12 (circles) of vancomycin on bacterial counts (log10 CFU/ml) in pouch exudates over time. Untreated controls are represented by filled squares. The error bars indicate standard deviation.
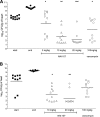
FIG. 2.
Efficacies of NAI-107 and vancomycin in reducing the bacterial load in heart vegetations in a model of rat endocarditis caused by S. aureus 1524. “Start” and “end” indicate bacterial loads at the start (17 h after infection) and end (5 days) of therapy, respectively. Each symbol represents the viable bacterial counts determined in each animal, while the horizontal lines represent the average value for each group. (A) NAI-107 at 5, 10, and 20 mg/kg i.v. q12 compared to 100 mg/kg i.m. q12 of vancomycin. Three animals treated with 5 mg/kg i.v. and two treated with 20 mg/kg i.v. of NAI-107 and two animals treated with 100 mg/kg i.m. of vancomycin were excluded from the statistical analysis due to incorrect placement of the catheter. *, P < 0.05 compared to start of therapy; **, _P_ < 0.001 compared to start of therapy and NAI-107 at 5 mg/kg and _P_ < 0.01 compared to vancomycin; ***, _P_ < 0.001 compared to start of therapy, NAI-107 at 5 mg/kg, and vancomycin. (B) NAI-107 at 10 mg/kg q12 versus 20 mg/kg i.v. q24 compared to 100 mg/kg i.m. q12 of vancomycin. One animal treated with 10 mg/kg i.v. of NAI-107 was excluded from the statistical evaluation due to incorrect placement of the catether. *, _P_ < 0.01 and _P_ > 0.001 compared to control groups and vancomycin, respectively; **, P < 0.001 compared to start and end of therapy.
Similar articles
- Pharmacological properties of NAI-603, a well-tolerated semisynthetic derivative of ramoplanin.
Jabes D, Brunati C, Candiani G, Riva S, Romanó G, Maffioli S, Rossi R, Simone M, Gaspari E, Donadio S. Jabes D, et al. Antimicrob Agents Chemother. 2014;58(4):1922-9. doi: 10.1128/AAC.01620-13. Epub 2014 Jan 13. Antimicrob Agents Chemother. 2014. PMID: 24419352 Free PMC article. - Synergistic activity of ceftobiprole and vancomycin in a rat model of infective endocarditis caused by methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus.
Fernandez J, Abbanat D, Shang W, He W, Amsler K, Hastings J, Queenan AM, Melton JL, Barron AM, Flamm RK, Lynch AS. Fernandez J, et al. Antimicrob Agents Chemother. 2012 Mar;56(3):1476-84. doi: 10.1128/AAC.06057-11. Epub 2012 Jan 9. Antimicrob Agents Chemother. 2012. PMID: 22232278 Free PMC article. - Daptomycin is effective in treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus.
Marco F, de la Mària CG, Armero Y, Amat E, Soy D, Moreno A, del Río A, Almela M, Mestres CA, Gatell JM, Jiménez de Anta MT, Miró JM; Hospital Clinic Experimental Endocarditis Study Group. Marco F, et al. Antimicrob Agents Chemother. 2008 Jul;52(7):2538-43. doi: 10.1128/AAC.00510-07. Epub 2008 Apr 21. Antimicrob Agents Chemother. 2008. PMID: 18426900 Free PMC article. - The oxadiazole antibacterials.
Janardhanan J, Chang M, Mobashery S. Janardhanan J, et al. Curr Opin Microbiol. 2016 Oct;33:13-17. doi: 10.1016/j.mib.2016.05.009. Epub 2016 May 27. Curr Opin Microbiol. 2016. PMID: 27239942 Free PMC article. Review. - Linezolid: a review of its use in the management of serious gram-positive infections.
Perry CM, Jarvis B. Perry CM, et al. Drugs. 2001;61(4):525-51. doi: 10.2165/00003495-200161040-00008. Drugs. 2001. PMID: 11324682 Review.
Cited by
- The Complex Role of Lactic Acid Bacteria in Food Detoxification.
Petrova P, Arsov A, Tsvetanova F, Parvanova-Mancheva T, Vasileva E, Tsigoriyna L, Petrov K. Petrova P, et al. Nutrients. 2022 May 12;14(10):2038. doi: 10.3390/nu14102038. Nutrients. 2022. PMID: 35631179 Free PMC article. Review. - Semisynthetic Macrocyclic Lipo-lanthipeptides Display Antimicrobial Activity Against Bacterial Pathogens.
Zhao X, Xu Y, Viel JH, Kuipers OP. Zhao X, et al. ACS Synth Biol. 2021 Aug 20;10(8):1980-1991. doi: 10.1021/acssynbio.1c00161. Epub 2021 Aug 4. ACS Synth Biol. 2021. PMID: 34347446 Free PMC article. - A Genome-Wide Analysis of Antibiotic Producing Genes in Streptomyces globisporus SP6C4.
Kim DR, Kwak YS. Kim DR, et al. Plant Pathol J. 2021 Aug;37(4):389-395. doi: 10.5423/PPJ.NT.03.2021.0047. Epub 2021 Aug 1. Plant Pathol J. 2021. PMID: 34365750 Free PMC article. - Correlational networking guides the discovery of unclustered lanthipeptide protease-encoding genes.
Xue D, Older EA, Zhong Z, Shang Z, Chen N, Dittenhauser N, Hou L, Cai P, Walla MD, Dong SH, Tang X, Chen H, Nagarkatti P, Nagarkatti M, Li YX, Li J. Xue D, et al. Nat Commun. 2022 Mar 28;13(1):1647. doi: 10.1038/s41467-022-29325-1. Nat Commun. 2022. PMID: 35347143 Free PMC article. - Structure and tRNA Specificity of MibB, a Lantibiotic Dehydratase from Actinobacteria Involved in NAI-107 Biosynthesis.
Ortega MA, Hao Y, Walker MC, Donadio S, Sosio M, Nair SK, van der Donk WA. Ortega MA, et al. Cell Chem Biol. 2016 Mar 17;23(3):370-380. doi: 10.1016/j.chembiol.2015.11.017. Epub 2016 Feb 11. Cell Chem Biol. 2016. PMID: 26877024 Free PMC article.
References
- Anonymous. 2009. Urgently needed: new antibiotics. Lancet 374:1868. - PubMed
- Baddour, L. M., et al. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:394-434. - PubMed
- Boakes, S., A. N. Appleyard, J. Cortés, and M. J. Dawson. 2010. Organization of the biosynthetic genes encoding deoxyactagardine B (DAB), a new lantibiotic produced by Actinoplanes liguriae NCIMB41362. J. Antibiot. (Tokyo). 63:351-358. - PubMed
- Bossaer, J. B., P. D. Hall, and E. Garrett-Mayer. 2010. Incidence of vancomycin-resistant enterococci (VRE) infection in high-risk febrile neutropenic patients colonized with VRE. Support Care Cancer 19:231-237. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical