Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis - PubMed (original) (raw)
Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis
Kisha Piggott et al. Circulation. 2011.
Abstract
Background: Giant cell arteritis is a granulomatous vasculitis of the aorta and its branches that causes blindness, stroke, and aortic aneurysm. CD4 T cells are key pathogenic regulators, instructed by vessel wall dendritic cells to differentiate into vasculitic T cells. The unique pathways driving this dendritic cell-T-cell interaction are incompletely understood, but may provide novel therapeutic targets for a disease in which the only established therapy is long-term treatment with high doses of corticosteroids.
Methods and results: Immunohistochemical and gene expression analyses of giant cell arteritis-affected temporal arteries revealed abundant expression of the NOTCH receptor and its ligands, Jagged1 and Delta1. Cleavage of the NOTCH intracellular domain in wall-infiltrating T cells indicated ongoing NOTCH pathway activation in large-vessel vasculitis. NOTCH activation did not occur in small-vessel vasculitis affecting branches of the vasa vasorum tree. We devised 2 strategies to block NOTCH pathway activation: γ-secretase inhibitor treatment, preventing nuclear translocation of the NOTCH intracellular domain, and competing for receptor-ligand interactions through excess soluble ligand, Jagged1-Fc. In a humanized mouse model, NOTCH pathway disruption had strong immunosuppressive effects, inhibiting T-cell activation in the early and established phases of vascular inflammation. NOTCH inhibition was particularly effective in downregulating Th17 responses, but also markedly suppressed Th1 responses.
Conclusions: Blocking NOTCH signaling depleted T cells from the vascular infiltrates, implicating NOTCH- NOTCH ligand interactions in regulating T-cell retention and survival in vessel wall inflammation. Modulating the NOTCH signaling cascade emerges as a promising new strategy for immunosuppressive therapy of large-vessel vasculitis.
Figures
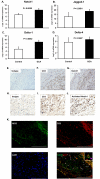
Figure 1. Activated NOTCH1 is abundant in GCA arteries
RNA was isolated from temporal artery biopsies, which either had no evidence for inflammation (Control) or showed granulomatous infiltrates typical of GCA (GCA) (n=4). Expression levels of NOTCH1 (A), Jagged1 (B), Delta1 (C) and Delta 4 (D) transcripts were quantified by RT-PCR. Data shown are mean ± SD. Paraffin-embedded temporal artery specimens affected by GCA were stained with anti-human CD3 (F and I), anti-human NOTCH1 (G), or antibodies to the cleaved intracellular domain of the NOTCH1 receptor (J). Isotype antibodies served as control (E and H). Activated NOTCH1 and CD3 were visualized by immunofluorescence using Alexa 488-labeled (green) and Alexa 546-labeled (red) antibodies, respectively (K). Colocalization of NICD and CD3 resulted in a yellow cellular stain (merge). Original magnification, E: ×100, scale bar = 500 μm; F, G, H, K: ×200, scale bar = 200 μm; I, J: ×400, scale bar = 100 μm.
Figure 2. Tissue-infiltrating T cells in small vessel vasculitis are NOTCH1 negative
Paraffin-embedded temporal artery specimens from patients with small vessel vasculitis in the vasa vasorum branches were stained with anti-human CD3 (A and B) or anti-human NOTCH1 (C). Original magnification, A: ×100, scale bar = 500 μm; B, C: ×400, scale bar = 100 μm.
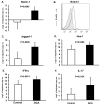
Figure 3. Expression of NOTCH1 receptor and the proinflammatory cytokines IL-17 and IFN-γ by peripheral T cells in GCA
Peripheral blood mononuclear cells were isolated from eight GCA patients and eight healthy controls (Control). NOTCH1 (A), Jagged1 (C) and Hes-1 (D), IL-17 (E) and IFN-γ (F) specific transcripts were quantified by RT-PCR. Data are shown as mean ± SD. Surface expression of NOTCH1 (black line) on CD4 T-cells was determined by flow cytometry (B). Shaded histogram =isotype control.
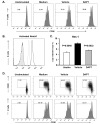
Figure 4. NOTCH pathway blockade dampens T-cell responses
Freshly isolated CD4 T-cells were stimulated with anti-CD3/CD28 mAbs (1 μg/ml) in the presence or absence of the γ-secretase inhibitor DAPT (10 μM) or vehicle control. CFSE dilution was evaluated by flow cytometry at 72 h (A). CD4 T-cells were stimulated for 24 h with anti-CD3/CD28 (5 μg/ml) in the presence of DAPT (light grey) or vehicle (black line) and analyzed for the cleaved intracellular domain of the NOTCH1 receptor (B). Shaded histogram = isotype control. Hes-1 transcripts were quantified by RT-PCR (C). CFSE-labeled T-cells were cultured with DC and anti-CD3 (1 μg/ml), in the presence or absence of DAPT or vehicle. On day 3 cells were stained for CD4 and CD25 (upper panels) and proliferation was assessed by flow cytometry using CD4 gates (upper and lower panels) (D). Percentage of cells that have divided are indicated. Data shown are representative of at least 6 independent experiments.
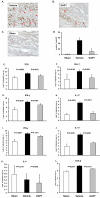
Figure 5. Treatment with a γ-secretase inhibitor suppresses vessel wall inflammation
For each experimental series segments from a human axillary artery were implanted into 3 SCID mice. The sham group received PBS (day 7) followed by intravenous adoptive transfer of PBMC (day 8). The vehicle group was injected with LPS (3 μg/mouse; day 7) followed by vehicle-pretreated PBMC (day 8) and vehicle injection i.p. the next day. DAPT treatment involved LPS injection (day 7) and adoptive transfer of DAPT-pretreated PBMC (day 8) followed by an injection of DAPT (1mg; day 9). PBMC derived from either healthy donors (A-H) or from patients with biopsy-proven GCA (I-K). Arteries were explanted 1 week later. Human T-cell infiltrates (brown color) in sham (A), vehicle- (B) or DAPT-treated (C) arteries were stained with rabbit anti-human CD3 Ab (A-C). Magnification: ×200. Vessel-infiltrating T-cells were enumerated in at least 10 randomly chosen high-powered fields (D). TCR (E), Hes-1 (F), IFN-γ (G and I), IL-17 (H and J), IL-4 (K) and TGFβ (L) transcripts in the tissues were quantified by RT-PCR. Results from five independent experiments are shown as mean ± S.D.
Figure 6. Soluble Jagged1 ligand inhibits T cell activation and abrogates vessel wall inflammation
CFSE-labeled CD4 T cells were incubated with αCD3/CD28 antibodies in the presence or absence of Jagged1-Fc or control Fc for 72 h. Cells were stained with anti-CD25 and proliferation was analyzed by flow cytometry (A). The effect of increasing concentrations of Jagged1-Fc or control-Fc on T-cell proliferation was measured at 72 h by 3H-thymidine incorporation in triplicate cultures (B). For in vivo experiments, CD4 T cells pre-incubated with Jagged1-Fc or control Fc were adoptively transferred into SCID-chimeras 8 days after implantation of human arteries as described in Figure 5. Additional doses of Jagged1-Fc or control Fc were given 24 and 48 h later via i.p. injection. Arterial grafts explanted one week later were analyzed by RT-PCR for Hes-1 (C), TCR (D), IFN-γ (E), IL-17 (F), and IL-4 (G) gene expression. Data are shown as mean ± S.D.
Figure 7. NOTCH inhibition attenuates ongoing T-cell inflammatory responses in the vessel wall
Vascular inflammation was induced in human artery-SCID chimeras by LPS injection and adoptive transfer of PBMC as described in Figure 5. Five days after initiating vessel wall inflammation 1 mg DAPT or vehicle was administered by i.p. injection, with three subsequent doses given 24, 48, and 72 h later. Grafts were explanted on day 20 postimplantation and markers of human CD4 T-cell infiltration were quantified by RT-PCR for TCR (A) and CCR6 (B) transcripts. Inflammatory burden and in situ T-cell activation was evaluated by measuring IFN-γ (C) and IL-17 (D) by RT-PCR. Hes-1 expression (E) in tissue grafts was measured to assess ongoing NOTCH signaling in tissue-infiltrating cells. Smooth muscle cell damage was examined by quantifying the expression of smoothelin (F), a marker of smooth muscle cell viability and function. Data from five independent experiments are shown as mean ± S.D.
Similar articles
- Hepatocytes contribute to immune regulation in the liver by activation of the Notch signaling pathway in T cells.
Burghardt S, Erhardt A, Claass B, Huber S, Adler G, Jacobs T, Chalaris A, Schmidt-Arras D, Rose-John S, Karimi K, Tiegs G. Burghardt S, et al. J Immunol. 2013 Dec 1;191(11):5574-82. doi: 10.4049/jimmunol.1300826. Epub 2013 Oct 18. J Immunol. 2013. PMID: 24140644 - Small interfering RNA-mediated knockdown of notch ligands in primary CD4+ T cells and dendritic cells enhances cytokine production.
Stallwood Y, Briend E, Ray KM, Ward GA, Smith BJ, Nye E, Champion BR, McKenzie GJ. Stallwood Y, et al. J Immunol. 2006 Jul 15;177(2):885-95. doi: 10.4049/jimmunol.177.2.885. J Immunol. 2006. PMID: 16818743 - Detrimental effects of Notch1 signaling activated by cadmium in renal proximal tubular epithelial cells.
Fujiki K, Inamura H, Matsuoka M. Fujiki K, et al. Cell Death Dis. 2014 Aug 14;5(8):e1378. doi: 10.1038/cddis.2014.339. Cell Death Dis. 2014. PMID: 25118938 Free PMC article. - Innate and Adaptive Immunity in Giant Cell Arteritis.
Akiyama M, Ohtsuki S, Berry GJ, Liang DH, Goronzy JJ, Weyand CM. Akiyama M, et al. Front Immunol. 2021 Feb 25;11:621098. doi: 10.3389/fimmu.2020.621098. eCollection 2020. Front Immunol. 2021. PMID: 33717054 Free PMC article. Review. - Cytokines, growth factors and proteases in medium and large vessel vasculitis.
Weyand CM, Watanabe R, Zhang H, Akiyama M, Berry GJ, Goronzy JJ. Weyand CM, et al. Clin Immunol. 2019 Sep;206:33-41. doi: 10.1016/j.clim.2019.02.007. Epub 2019 Feb 14. Clin Immunol. 2019. PMID: 30772599 Free PMC article. Review.
Cited by
- Macrophages in vascular inflammation--From atherosclerosis to vasculitis.
Shirai T, Hilhorst M, Harrison DG, Goronzy JJ, Weyand CM. Shirai T, et al. Autoimmunity. 2015 May;48(3):139-51. doi: 10.3109/08916934.2015.1027815. Epub 2015 Mar 26. Autoimmunity. 2015. PMID: 25811915 Free PMC article. Review. - VEGF combined with DAPT promotes tissue regeneration and remodeling in vascular grafts.
Yang T, Li G, Li X, Wei B, Su H, Liu W, Guo S, Yang N, Xu T, Duan C. Yang T, et al. Regen Biomater. 2023 Oct 13;10:rbad088. doi: 10.1093/rb/rbad088. eCollection 2023. Regen Biomater. 2023. PMID: 37899954 Free PMC article. - Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis.
Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM. Zhang H, et al. Circulation. 2018 May 1;137(18):1934-1948. doi: 10.1161/CIRCULATIONAHA.117.030423. Epub 2017 Dec 18. Circulation. 2018. PMID: 29254929 Free PMC article. - Characterization of the Notch pathway in nasal polyps of patients with chronic rhinosinusitis: A pilot study.
Aquila G, Alaimo A, Marracino L, Martino V, Camponogara F, Vieceli Dalla Sega F, Fortini F, Pannuti A, Zanotti C, Malagutti N, Pelucchi S, Rizzo P. Aquila G, et al. Physiol Rep. 2022 Aug;10(16):e15403. doi: 10.14814/phy2.15403. Physiol Rep. 2022. PMID: 36029197 Free PMC article. - Pharmacological inhibition of Notch signaling regresses pre-established abdominal aortic aneurysm.
Sharma N, Dev R, Ruiz-Rosado JD, Partida-Sanchez S, Guerau-de-Arellano M, Dhakal P, Kuivaniemi H, Hans CP. Sharma N, et al. Sci Rep. 2019 Sep 17;9(1):13458. doi: 10.1038/s41598-019-49682-0. Sci Rep. 2019. PMID: 31530833 Free PMC article.
References
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. - PubMed
- Weyand CM, Tetzlaff N, Bjornsson J, Brack A, Younge B, Goronzy JJ. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40:19–26. - PubMed
- Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM. Toll-like receptors in giant cell arteritis. Clin Immunol. 2005;115:38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI57266/AI/NIAID NIH HHS/United States
- R01 EY011916-14/EY/NEI NIH HHS/United States
- R01 AI044142/AI/NIAID NIH HHS/United States
- R01 EY011916-13/EY/NEI NIH HHS/United States
- R01 AI44142/AI/NIAID NIH HHS/United States
- R01 AR042527-16/AR/NIAMS NIH HHS/United States
- R01 EY011916-11A1/EY/NEI NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- R01 AR042527/AR/NIAMS NIH HHS/United States
- U19 AI057266-01/AI/NIAID NIH HHS/United States
- R01 AI044142-12/AI/NIAID NIH HHS/United States
- R01 EY11916/EY/NEI NIH HHS/United States
- P01 HL058000/HL/NHLBI NIH HHS/United States
- R01 AI044142-10/AI/NIAID NIH HHS/United States
- R01 AR42527/AR/NIAMS NIH HHS/United States
- P01 HL058000-05/HL/NHLBI NIH HHS/United States
- R01 AI044142-11/AI/NIAID NIH HHS/United States
- R01 AR042527-18/AR/NIAMS NIH HHS/United States
- R01 EY011916-12/EY/NEI NIH HHS/United States
- R01 EY011916/EY/NEI NIH HHS/United States
- R01 AR042527-17/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials