Dichotomous regulation of GVHD through bidirectional functions of the BTLA-HVEM pathway - PubMed (original) (raw)
Dichotomous regulation of GVHD through bidirectional functions of the BTLA-HVEM pathway
Yukimi Sakoda et al. Blood. 2011.
Abstract
B and T lymphocyte attenuator (BTLA) is a co-inhibitory receptor that interacts with herpesvirus entry mediator (HVEM), and this interaction regulates pathogenesis in various immunologic diseases. In graft-versus-host disease (GVHD), BTLA unexpectedly mediates positive effects on donor T-cell survival, whereas immunologic mechanisms of this function have yet to be explored. In this study, we elucidated a role of BTLA in GVHD by applying the newly established agonistic anti-BTLA monoclonal antibody that stimulates BTLA signal without antagonizing BTLA-HVEM interaction. Our results revealed that provision of BTLA signal inhibited donor antihost T-cell responses and ameliorated GVHD with a successful engraftment of donor hematopoietic cells. These effects were dependent on BTLA signal into donor T cells but neither donor non-T cells nor recipient cells. On the other hand, expression of BTLA mutant lacking an intracellular signaling domain restored impaired survival of BTLA-deficient T cells, suggesting that BTLA also serves as a ligand that delivers HVEM prosurvival signal in donor T cells. Collectively, current study elucidated dichotomous functions of BTLA in GVHD to serve as a costimulatory ligand of HVEM and to transmit inhibitory signal as a receptor.
Figures
Figure 1
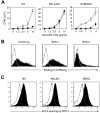
Generation of agonistic, but not antagonistic, anti-BTLA mAb BYK-1. (A) T cells isolated from WT, _BTLA_-KO, or _HVEM_-KO mice were stimulated with indicated doses of anti-CD3 mAb in the presence of 20 μg/mL immobilized control rat IgG (□) or BYK-1 (■). Proliferative activity was assessed by 3H-thymidine incorporation during the last 18 hours of a 3-day culture. (B) BTLA-expressing CHO cells were cultured with HVEM-mouse Ig fusion protein in the presence of 10 μg control Ig, BYK-1, or BYK-2 (filled histogram). Background staining without HVEM-Ig is also shown (open histogram). Binding of HVEM-Ig fusion protein was detected by a staining with fluorescein isothiocyanate–conjugated antimouse Ig Ab. (C) CD4+ T cells isolated from B6, BALB/c, or DBA/2 mice were stimulated with 1 μg/mL antimouse CD3 mAb. After 48 hours, T cells were stained with BYK-1 (filled histogram) or control Ig (open histogram), and analyzed by flow cytometry. Data are representative of 3 independent experiments.
Figure 2
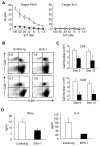
Inhibition of donor antihost alloresponses by BYK-1 treatment. (A-C) BDF1 recipient mice were injected intravenously with 5 × 107 donor B6 spleen cells. The recipient mice were treated intraperitoneally with 200 μg of BYK-1 (■) or control Ig (□) on days 0, 3, and 6. (A) On day 9, recipient spleen cells were harvested and assessed for CTL activity against P815 (H-2d) and EL4 (H-2b) cells by a standard 4-hours 51Cr releasing assay. (B) On day 9, recipient spleen cells were stained with anti-H-2Kd mAb, together with either anti-CD4 or anti-CD8 mAb, and analyzed by flow cytometry. Percentages of donor CD4+ or CD8+ T cells (upper left quadrant) in the recipient spleen are shown. The experiment was repeated more than 3 times, and representative data are shown. P < .05 (control Ig vs BYK-1). (C) On days 9 and 12, absolute numbers of donor CD4+ and CD8+ T cells in the recipient spleen were assessed. Each column represents average plus or minus SD of donor T-cell number after the treatment with control Ig (white bar) or BYK-1 (black bar). (D) BDF1 recipient mice were exposed to irradiation (9 Gy) and subsequently injected intravenously with 5 × 106 donor B6 spleen cells. The recipient mice were injected intraperitoneally with 200 μg BYK-1 (black bar) or control Ig (white bar) on days 0 and 3. On day 6, mesenteric lymph node cells harvested from the recipient mice were standardized for numbers of CD4+ T cells (5 × 104 per well) and stimulated in vitro with 5 μg/mL immobilized anti-CD3 mAb and 2 μg/mL anti-CD28 mAb. After 24 hours, the culture supernatants were harvested, and the levels of interferon-γ (left panel) and IL-4 (right panel) were measured by enzyme-linked immunosorbent assay. Each column represents average plus or minus SD. P < .05 (control Ig vs BYK-1). Data are representative of 3 independent experiments.
Figure 3
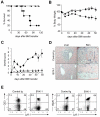
Therapeutic effects of BYK-1 in GVHD induced by MHC-matched, minor histocompatibility antigen-mismatched BMT. On day 0, B6 recipient mice were exposed to lethal irradiation (12 Gy) and transferred intravenously with 5 × 106 T cell-depleted BM cells together with 3 × 106 spleen plus lymph node cells isolated from C3H.SW donor mice. The recipient mice were treated intraperitoneally with 200 μg BYK-1 (▲) or control Ig (●) every 5 days from day 0 to day 25. As non-GVHD control, a group of mice were transferred with T cell–depleted BM cells alone (○). Survival of recipient mice (A), changes in body weight (B), and GVHD clinical scores (C) were monitored after BMT. (D) Pathologic features of recipient liver and skin were examined by hematoxylin and eosin staining (original magnification ×400 in the liver and ×100 in the skin). (E) Spleen cells of the recipient mice that had survived GVHD more than 100 days by BYK-1 treatment were assessed for the expression of Ly9.1 (donor cell marker) and CD3 or B220 by flow cytometry. Data are representative of 3 independently repeated experiments (n = 5 per group).
Figure 4
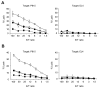
BTLA expressed on donor T cells as an essential target for inhibitory effects of BYK-1 in GVHD. (A) BDF1 mice were injected intravenously with 5 × 107 B6 WT or _BTLA_-KO mouse spleen cells on day 0 and treated intraperitoneally with 200 μg BYK-1 or control Ig on days 0, 3, and 6 in the following combinations: transfer of WT donor cells and control Ig treatment (□), transfer of WT donor cells and BYK-1 treatment (■), transfer of _BTLA_-KO donor cells and control Ig treatment (○), and transfer of _BTLA_-KO donor cells and BYK-1 treatment (●). On day 9, recipient spleen cells were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b) cells. (B) BDF1 mice were injected with mixture of T cells (2 × 107 cells) and non-T cells (4 × 107 cells) purified from either B6 WT or _BTLA_-KO mice and treated intraperitoneally with BYK-1 or control Ig on days 0, 3, and 6 in the following combinations: transfer of WT T cells plus _BTLA_-KO non-T cells followed by control Ig treatment (□), transfer of WT T cells plus _BTLA_-KO non-T cells followed by BYK-1 treatment (■), transfer of _BTLA_-KO T cells plus WT non-T cells followed by control Ig treatment (○), and transfer of _BTLA_-KO T cells plus WT non-T cells followed by BYK-1 treatment (●). On day 9, recipient spleen cells were harvested and analyzed for CTL activity against P815 (H-2d) and EL4 (H-2b). CTL level against P815 in BYK-1-treated WT T cells plus _BTLA_-KO non-T cells (■) was significantly lower than control Ig-treated _BTLA_-KO T cells plus WT non-T cells (○) at an effector/target (E/T) ratio of 100, 50, 25, and 12 (P < .05). Data are representative of 3 independently repeated experiments.
Figure 5
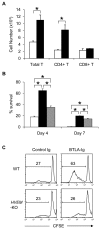
BTLA functions as a ligand to deliver prosurvival signal in GVHD. (A) Activated _BTLA_-KO B6 T cells expressing BTLAΔCY-GFP (black bar) or GFP alone (white bar) were transferred intravenously into sublethally (6 Gy)-irradiated BDF1 mice (7 × 105 cells/mouse). After 24 hours, spleen was harvested from the recipient mice and the numbers of GFP-positive donor T cells (total, CD4+, and CD8+) were assessed by flow cytometry. *P < .05. (B) Activated _BTLA_-KO B6 T cells expressing BTLAΔCY-GFP (black bar), wild-type BTLA-GFP (gray bar), and GFP alone (white bar) were incubated in vitro. After 4 and 7 days, the number of live GFP-positive cells was counted and percentage survival is shown as average plus or minus SD of triplicate wells. *P < .05. (C) T cells isolated from spleen of WT or _HVEM_-KO mice were labeled with CFSE and stimulated with 2 μg/mL anti-CD3 mAb in the presence of 10 μg/mL immobilized BTLA-Ig or control Ig. After 3 days, the intensity of CFSE of the culture cells was analyzed by flow cytometry. The numbers in panels indicate percentage of cells undergone more than one division.
Similar articles
- The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation.
Cai G, Freeman GJ. Cai G, et al. Immunol Rev. 2009 May;229(1):244-58. doi: 10.1111/j.1600-065X.2009.00783.x. Immunol Rev. 2009. PMID: 19426226 Review. - T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment.
Cheung TC, Oborne LM, Steinberg MW, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. Cheung TC, et al. J Immunol. 2009 Dec 1;183(11):7286-96. doi: 10.4049/jimmunol.0902490. Epub 2009 Nov 13. J Immunol. 2009. PMID: 19915044 Free PMC article. - Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells.
Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW. Tao R, et al. J Immunol. 2008 May 15;180(10):6649-55. doi: 10.4049/jimmunol.180.10.6649. J Immunol. 2008. PMID: 18453584 - Unconventional ligand activation of herpesvirus entry mediator signals cell survival.
Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. Cheung TC, et al. Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6244-9. doi: 10.1073/pnas.0902115106. Epub 2009 Mar 30. Proc Natl Acad Sci U S A. 2009. PMID: 19332782 Free PMC article. - Modulation of T cell proliferation through the LIGHT-HVEM-BTLA cosignaling pathway.
Cheung TC. Cheung TC. Recent Pat DNA Gene Seq. 2009;3(3):177-82. doi: 10.2174/187221509789318342. Recent Pat DNA Gene Seq. 2009. PMID: 19702559 Review.
Cited by
- Distinct characteristics of BTLA/HVEM axis expression on Tregs and its impact on the expansion and attributes of Tregs in patients with active pulmonary tuberculosis.
Tang P, Shen X, Gao J, Zhang J, Feng Y, Zhang J, Huang Z, Wang X. Tang P, et al. Front Cell Infect Microbiol. 2024 Sep 25;14:1437207. doi: 10.3389/fcimb.2024.1437207. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39386167 Free PMC article. - Beyond the anti-PD-1/PD-L1 era: promising role of the BTLA/HVEM axis as a future target for cancer immunotherapy.
Sordo-Bahamonde C, Lorenzo-Herrero S, Granda-Díaz R, Martínez-Pérez A, Aguilar-García C, Rodrigo JP, García-Pedrero JM, Gonzalez S. Sordo-Bahamonde C, et al. Mol Cancer. 2023 Aug 30;22(1):142. doi: 10.1186/s12943-023-01845-4. Mol Cancer. 2023. PMID: 37649037 Free PMC article. Review. - Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts.
Wang Y, Zhang H, Liu C, Wang Z, Wu W, Zhang N, Zhang L, Hu J, Luo P, Zhang J, Liu Z, Peng Y, Liu Z, Tang L, Cheng Q. Wang Y, et al. J Hematol Oncol. 2022 Aug 17;15(1):111. doi: 10.1186/s13045-022-01325-0. J Hematol Oncol. 2022. PMID: 35978433 Free PMC article. Review. - Post-hematopoietic stem cell transplantation relapse: Role of checkpoint inhibitors.
Roshandel E, Tavakoli F, Parkhideh S, Akhlaghi SS, Ardakani MT, Soleimani M. Roshandel E, et al. Health Sci Rep. 2022 Mar 8;5(2):e536. doi: 10.1002/hsr2.536. eCollection 2022 Mar. Health Sci Rep. 2022. PMID: 35284650 Free PMC article. Review. - HVEM Promotes the Osteogenesis of allo-MSCs by Inhibiting the Secretion of IL-17 and IFN-γ in Vγ4T Cells.
He L, Xiao J, Song L, Zhou R, Rong Z, He W, Dai F. He L, et al. Front Immunol. 2021 Jun 23;12:689269. doi: 10.3389/fimmu.2021.689269. eCollection 2021. Front Immunol. 2021. PMID: 34248977 Free PMC article.
References
- Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71(7):1065–1068. - PubMed
- Chambers CA, Allison JP. Co-stimulation in T-cell responses. Curr Opin Immunol. 1997;9(3):396–404. - PubMed
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. - PubMed
- Subudhi SK, Alegre ML, Fu YX. The balance of immune responses: costimulation versus coinhibition. J Mol Med. 2005;83(3):193–202. - PubMed
- Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials