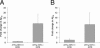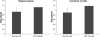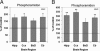Neprilysin-2 is an important β-amyloid degrading enzyme - PubMed (original) (raw)
Neprilysin-2 is an important β-amyloid degrading enzyme
Daniel Hafez et al. Am J Pathol. 2011 Jan.
Abstract
Proteases that degrade the amyloid-β peptide (Aβ) are important in protecting against Alzheimer's disease (AD), and understanding these proteases is critical to understanding AD pathology. Endopeptidases sensitive to inhibition by thiorphan and phosphoramidon are especially important, because these inhibitors induce dramatic Aβ accumulation (∼30- to 50-fold) and pathological deposition in rodents. The Aβ-degrading enzyme neprilysin (NEP) is the best known target of these inhibitors. However, genetic ablation of NEP results in only modest increases (∼1.5- to 2-fold) in Aβ, indicating that other thiorphan/phosphoramidon-sensitive endopeptidases are at work. Of particular interest is the NEP homolog neprilysin 2 (NEP2), which is thiorphan/phosphoramidon-sensitive and degrades Aβ. We investigated the role of NEP2 in Aβ degradation in vivo through the use of gene knockout and transgenic mice. Mice deficient for the NEP2 gene showed significant elevations in total Aβ species in the hippocampus and brainstem/diencephalon (∼1.5-fold). Increases in Aβ accumulation were more dramatic in NEP2 knockout mice crossbred with APP transgenic mice. In NEP/NEP2 double-knockout mice, Aβ levels were marginally increased (∼1.5- to 2-fold), compared with NEP(-/-)/NEP2(+/+) controls. Treatment of these double-knockout mice with phosphoramidon resulted in elevations of Aβ, suggesting that yet other NEP-like Aβ-degrading endopeptidases are contributing to Aβ catabolism.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure S1
Examples of genotyping assays for the NEP2/NEP knockout and APPtg mice. UV images of ethidium bromide-stained agarose electrophoresis gels showing DNA PCR products using specific primers (see Methods section) to amplify genomic template DNA extracted from mouse ear clippings.
Figure S2
Aβ levels in the cerebral cortex of APPtg mice in the presence and absence of the NEP2 gene. Relative levels of Aβ42 (A) and Aβ40 (B) measured by specific ELISA. Data are presented as the fold change compared with the average value from APPtg/NEP2+/+ control mice. Values are means ± SEM.
Figure S3
Analysis of intracellular Aβ accumulation in the presence and absence of the NEP2 gene. Representative fluorescent images of immunohistochemistry staining of Aβ from sagittal brain sections from APPtg/NEP2+/+ (A, B) and APPtg/NEP2−/− (C, D) mice from the hippocampus (A, C) and cerebral cortex (B, D). Quantitation of the % area staining positive for Aβ in the hippocampus (E) and cerebral cortex (F) from APPtg/NEP2+/+ (n = 4) and APPtg/NEP2−/− (n = 5) mice. Values are averages ± SEM.
Figure S4
Real-time PCR analysis of NEP2 expression on APP transgenic and non-transgenic mice. Coding DNA samples from mouse brain tissues (2 months old) were analyzed for NEP2 gene expression using the ΔΔCt-method (compared with cyclophilin expression) of quantitative real-time PCR. Data are presented as the mean of arbitrary expression units (resultant) ± SEM.
Figure 1
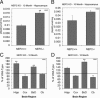
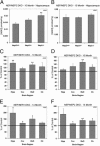
Amyloid β (Aβ) levels are increased in NEP2 knockout (KO) mice. Total Aβ42 (A) and Aβ40 (B) levels in the hippocampus of 10-month-old NEP2 wild-type (+/+) (n = 9) and NEP2 KO (−/−) (n = 7) mice. Relative levels of Aβ42 (C) and Aβ40 (D) are reported as a percentage of wild-type mice from hippocampus (Hipp), cerebral cortex (Ccx), brainstem/diencephalon (BsD), and cerebellum (Cb). Values are averages ± SEM. *P < 0.05, **P < 0.01.
Figure 2
Aβ40 levels are increased in the absence of NEP2 in APPtg mice. Total Aβ42 (A) and Aβ40 (B) levels in 10-month-old APPtg mice in the presence (+/+) (n = 4) and absence (−/−) (n = 5) of the NEP2 gene. Relative levels of Aβ42 (C) and Aβ40 (D) are reported as a percentage of APPtg/NEP2+/+ control mice from the hippocampus (Hipp), brainstem/diencephalon (BsD), and cerebellum (Cb). Values are averages ± SEM. **P < 0.01.
Figure 3
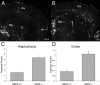
Aβ plaque deposition is increased in the absence of NEP2 in APPtg mice. Representative fluorescent images of immunohistochemistry staining of deposited Aβ (green) from sagittal brain sections from APPtg/NEP2+/+ mice (A) and APPtg/NEP2−/− mice (40×) (B). Arrowheads indicate examples of plaques. Cx, cerebral cortex; Hp, hippocampus. Quantitation of the % area staining positive for plaque deposition in the hippocampus (C) and cerebral cortex (D) from APPtg/NEP2+/+ mice (n = 4) and APPtg/NEP2−/− mice (n = 5). Values are averages ± SEM. *P < 0.05.
Figure 4
Murine NEP2α degrades Aβ42. Quantitative analysis of remaining Aβ42 in conditioned cell culture medium containing 50 pmol/L Aβ42 after incubation (5 hours) with HEK293T cells transfected with endopeptidase expressing plasmids (mNEP2α, hNEP) or control plasmid (green fluorescent protein, GFP) in the presence (+T) or absence of thiorphan (100 μmol/L) (n = 4). Values are averages ± SEM. **P < 0.01 compared with GFP.
Figure 5
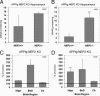
Aβ levels are increased in the absence of NEP2 in NEP KO mice. Total Aβ42 (A) and Aβ40 (B) in the hippocampus of 10-month-old NEP KO mice homozygous (n = 8), heterozygous (n = 5), and deficient (n = 10) for the NEP2 gene. Relative levels of Aβ42 (C, E) and Aβ40 (D, F) from 10-month-old (C, D) and 14-month-old (E, F) NEP/NEP2 DKO mice reported as a percentage of control (NEP−/−/NEP2+/+) mice from hippocampus (Hipp), cerebral cortex (Ccx), brainstem/diencephalon (BsD), and cerebellum (Cb). For the 14-month group, n = 9 for NEP2+/+ and n = 7 for NEP2−/−. Values are averages ± SEM. *P < 0.05, **P < 0.01.
Figure 6
Increased Aβ levels after phosphoramidon administration in NEP/NEP2 DKO mice. Relative levels of Aβ42 (A) and Aβ40 (B) in DKO mice (10 months old, n = 3) infused with phosphoramidon (i.c.v., 10 mmol/L, 0.11 μL/hr). Data are presented as the percentage of control (saline) Aβ levels from the hippocampus (Hipp), cerebral cortex (Ccx), brainstem/diencephalon (BsD), and cerebellum (Cb). Values are means ± SEM. *P < 0.05, **P < 0.01.
Similar articles
- Intranasal phosphoramidon increases beta-amyloid levels in wild-type and NEP/NEP2-deficient mice.
Hanson LR, Hafez D, Svitak AL, Burns RB, Li X, Frey WH 2nd, Marr RA. Hanson LR, et al. J Mol Neurosci. 2011 Mar;43(3):424-7. doi: 10.1007/s12031-010-9460-8. Epub 2010 Oct 13. J Mol Neurosci. 2011. PMID: 20941644 - NEP-like endopeptidases and Alzheimer's disease [corrected].
Marr RA, Spencer BJ. Marr RA, et al. Curr Alzheimer Res. 2010 May;7(3):223-9. doi: 10.2174/156720510791050849. Curr Alzheimer Res. 2010. PMID: 20088804 Review. - Neprilysin degrades both amyloid beta peptides 1-40 and 1-42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases.
Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K, Kiryu-Seo S, Kiyama H, Iwata H, Tomita T, Iwatsubo T, Saido TC. Shirotani K, et al. J Biol Chem. 2001 Jun 15;276(24):21895-901. doi: 10.1074/jbc.M008511200. Epub 2001 Mar 6. J Biol Chem. 2001. PMID: 11278416 - Amyloid-beta and Alzheimer's disease: the role of neprilysin-2 in amyloid-beta clearance.
Marr RA, Hafez DM. Marr RA, et al. Front Aging Neurosci. 2014 Aug 13;6:187. doi: 10.3389/fnagi.2014.00187. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25165447 Free PMC article. Review. - Human membrane metallo-endopeptidase-like protein degrades both beta-amyloid 42 and beta-amyloid 40.
Huang JY, Bruno AM, Patel CA, Huynh AM, Philibert KD, Glucksman MJ, Marr RA. Huang JY, et al. Neuroscience. 2008 Jul 31;155(1):258-62. doi: 10.1016/j.neuroscience.2008.05.006. Epub 2008 May 16. Neuroscience. 2008. PMID: 18571334 Free PMC article.
Cited by
- F-spondin gene transfer improves memory performance and reduces amyloid-β levels in mice.
Hafez DM, Huang JY, Richardson JC, Masliah E, Peterson DA, Marr RA. Hafez DM, et al. Neuroscience. 2012 Oct 25;223:465-72. doi: 10.1016/j.neuroscience.2012.07.038. Epub 2012 Jul 31. Neuroscience. 2012. PMID: 22863679 Free PMC article. - Neprilysin Inhibitors in Heart Failure: The Science, Mechanism of Action, Clinical Studies, and Unanswered Questions.
Bozkurt B, Nair AP, Misra A, Scott CZ, Mahar JH, Fedson S. Bozkurt B, et al. JACC Basic Transl Sci. 2022 Sep 7;8(1):88-105. doi: 10.1016/j.jacbts.2022.05.010. eCollection 2023 Jan. JACC Basic Transl Sci. 2022. PMID: 36777165 Free PMC article. Review. - NET amyloidogenic backbone in human activated neutrophils.
Pulze L, Bassani B, Gini E, D'Antona P, Grimaldi A, Luini A, Marino F, Noonan DM, Tettamanti G, Valvassori R, de Eguileor M. Pulze L, et al. Clin Exp Immunol. 2016 Mar;183(3):469-79. doi: 10.1111/cei.12730. Epub 2015 Dec 8. Clin Exp Immunol. 2016. PMID: 26462606 Free PMC article. - Neprilysin expression and functions in development, ageing and disease.
Nalivaeva NN, Zhuravin IA, Turner AJ. Nalivaeva NN, et al. Mech Ageing Dev. 2020 Dec;192:111363. doi: 10.1016/j.mad.2020.111363. Epub 2020 Sep 26. Mech Ageing Dev. 2020. PMID: 32987038 Free PMC article. Review. - MCF7 Spheroid Development: New Insight about Spatio/Temporal Arrangements of TNTs, Amyloid Fibrils, Cell Connections, and Cellular Bridges.
Pulze L, Congiu T, Brevini TAL, Grimaldi A, Tettamanti G, D'Antona P, Baranzini N, Acquati F, Ferraro F, de Eguileor M. Pulze L, et al. Int J Mol Sci. 2020 Jul 29;21(15):5400. doi: 10.3390/ijms21155400. Int J Mol Sci. 2020. PMID: 32751344 Free PMC article.
References
- Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. - PubMed
- Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H.J., Hama E., Sekine-Aizawa Y., Saido T.C. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. - PubMed
- Marr R.A., Spencer B.J. NEP-like Endopeptidases and Alzheimer's Disease. Curr Alzheimer Res. 2010;7:223–229. - PubMed
- Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H.J., Saido T.C. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases