Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats - PubMed (original) (raw)
Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats
Krishnan S Ramaswamy et al. J Physiol. 2011.
Abstract
The dystrophin–glycoprotein complex (DGC) provides an essential link from the muscle fibre cytoskeleton to the extracellular matrix. In dystrophic humans and mdx mice, mutations in the dystrophin gene disrupt the structure of the DGC causing severe damage to muscle fibres. In frog muscles, transmission of force laterally from an activated fibre to the muscle surface occurs without attenuation, but lateral transmission of force has not been demonstrated in mammalian muscles. A unique ‘yoke' apparatus was developed that attached to the epimysium of muscles midway between the tendons and enabled the measurement of lateral force. We now report that in muscles of young wild-type (WT) mice and rats, compared over a wide range of longitudinal forces, forces transmitted laterally showed little or no decrement. In contrast, for muscles of mdx mice and very old rats, forces transmitted laterally were impaired severely. Muscles of both mdx mice and very old rats showed major reductions in the expression of dystrophin. We conclude that during contractions, forces developed by skeletal muscles of young WT mice and rats are transmitted laterally from fibre to fibre through the DGC without decrement. In contrast, in muscles of dystrophic or very old animals, disruptions in DGC structure and function impair lateral transmission of force causing instability and increased susceptibility of fibres to contraction-induced injury.
Figures
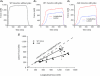
Figure 2. Comparison of the longitudinal and lateral transmission of forces in WT and mdx mice
Aa, a record of the maximum isometric tetanic force (_P_o) of an ATB muscle of a WT mouse. Note ATB and EDL muscles of WT and mdx mice and young and old WT rats display a very similar type of isometric force trace. Ab and c, records obtained following the attachment of the ‘yoke’ apparatus. The forces were measured during maximum stimulation of the motor nerve with the muscle at optimum length for the production of force (_L_o). Following the attachment of the ‘yoke’ apparatus, the longitudinal and lateral force traces of maximally activated WT (b) and mdx (c) muscles of mice displayed a slower initial rise in force and then a secondary even slower rise. After the attachment of the ‘yoke’, the _P_o values of the WT and mdx muscles were ∼25% lower than those of the muscles before the attachment of the ‘yoke’. Note the much closer relationship for the values for the lateral and longitudinal forces for the WT muscle than for the mdx muscle. B, relationship of longitudinal to lateral force transmission in anterior tibialis (ATB) muscles of mdx and WT mice.
Figure 1. The apparatus used to measure the longitudinal and lateral transmission of force
A, transmission of the force was measured from the proximal to the distal tendon to assess the longitudinal transmission of force. B, transmission of the force was measured from the proximal tendon laterally from the isometrically contracting fibres to the yoke. C, longitudinal and lateral force transmission in WT anterior tibialis anterior muscle. Note the closeness of the data to the line of identity between lateral and longitudinal force. D, diagram showing a cross-section of a skeletal muscle during the measurement of lateral transmission of forces. Note that throughout the contraction the fibres close to the surface of the muscle remain isometric between the proximal tendon and the yoke at an optimum length for force development. In contrast, the fibres toward the center of the muscle cross-section tend to shorten slightly and contribute less force than the fibres at optimum length.
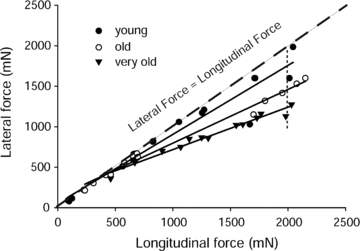
Figure 3. Lateral and longitudinal transmission of force for EDL muscles of young, old and very old rats
Values are given as the maximum isometric tetanic forces measured from proximal to distal tendon (longitudinal force) and from the yoke to the proximal tendon (lateral force) in cases of whole muscles as well as those from decreased sets of motor units.
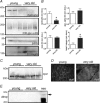
Figure 4. Expression of dystrophin–glycoprotein complex and integrin complex proteins in aged muscle membranes
One hundred micrograms of total hindlimb muscle membrane protein preparations from three young (12 month) and three very old rats (34–35 months) was analysed by SDS-PAGE and Western blotting (A) and quantified by densitometry (B) *P < 0.05 by Student's t test, data are means ±
s.e.m.
C and D, mislocalization of dysferlin and no upregulation of utrophin in aged muscle. While expression levels of dysferlin in membrane fractions is normal by Western blotting (C), immunolocalization of dysferlin (D) shows redistribution of dysferlin expression into cytoplasmic membrane compartments. E, no upregulation of utrophin was observed in aged muscle, although full length utrophin protein was detected in a positive control sample from neonatal muscle.
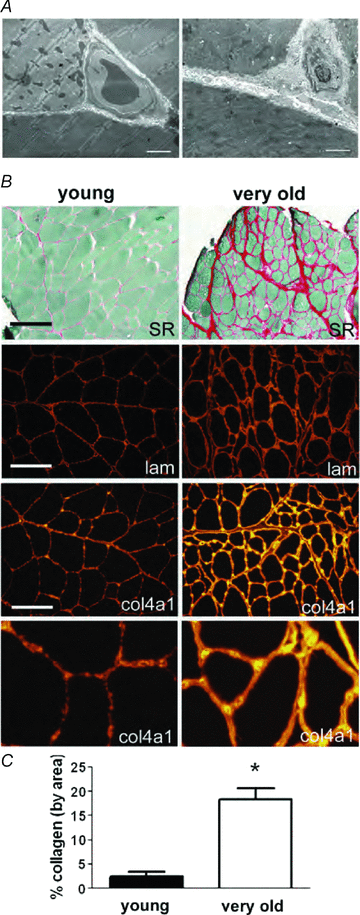
Figure 5. Increase in accumulation of interstitial connective tissue and basal lamina thickness in skeletal muscles of old rats
A, electron micrographs of three adjacent fibres of an EDL muscle showing thickening of the basal lamina in skeletal muscles of old compared to young rats. Three adjacent fibres from skeletal muscles of young (left) and old (right) rats are shown. Bar = 2 μm. B, increased abundance of fibrillar collagen and thickness of collagen IV and laminin in the basal lamina of muscle from very old rats. Picrosirius red staining followed by area analysis was used to quantify the fibrillar collagen expression in aged muscle. Immunolocalization of type IV collagen and laminin show increased thickness and staining intensity of the basal lamina. Bar = 200 μm, top two panels. Bar = 100 μm, immunofluorescence panels. Magnification of the bottom panels was increased identically for detailed comparative purposes. C, collagen composition. Two entire midbelly sections of picrosirius red stained sections from each muscle were quantified using a montage of 10× objective images, and averaged for each animal. *P < 0.005 by Student's t test, data are means ±
s.e.m.
, _n_= 3 animals in each.
Comment in
- On lateral transmission of force in active intact muscle.
Ranatunga KW. Ranatunga KW. J Physiol. 2011 Mar 1;589(Pt 5):1001. doi: 10.1113/jphysiol.2011.205187. J Physiol. 2011. PMID: 21486819 Free PMC article. No abstract available.
Similar articles
- Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice.
Stupka N, Plant DR, Schertzer JD, Emerson TM, Bassel-Duby R, Olson EN, Lynch GS. Stupka N, et al. J Physiol. 2006 Sep 1;575(Pt 2):645-56. doi: 10.1113/jphysiol.2006.108472. Epub 2006 Jun 22. J Physiol. 2006. PMID: 16793906 Free PMC article. - Muscle specific kinase protects dystrophic mdx mouse muscles from eccentric contraction-induced loss of force-producing capacity.
Trajanovska S, Ban J, Huang J, Gregorevic P, Morsch M, Allen DG, Phillips WD. Trajanovska S, et al. J Physiol. 2019 Sep;597(18):4831-4850. doi: 10.1113/JP277839. Epub 2019 Aug 18. J Physiol. 2019. PMID: 31340406 - Contractile efficiency of dystrophic mdx mouse muscle: in vivo and ex vivo assessment of adaptation to exercise of functional end points.
Capogrosso RF, Mantuano P, Cozzoli A, Sanarica F, Massari AM, Conte E, Fonzino A, Giustino A, Rolland JF, Quaranta A, De Bellis M, Camerino GM, Grange RW, De Luca A. Capogrosso RF, et al. J Appl Physiol (1985). 2017 Apr 1;122(4):828-843. doi: 10.1152/japplphysiol.00776.2015. Epub 2017 Jan 5. J Appl Physiol (1985). 2017. PMID: 28057817 - Twenty-one days of low-intensity eccentric training improve morphological characteristics and function of soleus muscles of mdx mice.
Pedrazzani PS, Araújo TOP, Sigoli E, da Silva IR, da Roza DL, Chesca DL, Rassier DE, Cornachione AS. Pedrazzani PS, et al. Sci Rep. 2021 Feb 11;11(1):3579. doi: 10.1038/s41598-020-79168-3. Sci Rep. 2021. PMID: 33574358 Free PMC article. - The dystrophin-glycoprotein complex in the prevention of muscle damage.
Gumerson JD, Michele DE. Gumerson JD, et al. J Biomed Biotechnol. 2011;2011:210797. doi: 10.1155/2011/210797. Epub 2011 Oct 5. J Biomed Biotechnol. 2011. PMID: 22007139 Free PMC article. Review.
Cited by
- Bioreactor development for skeletal muscle hypertrophy and atrophy by manipulating uniaxial cyclic strain: proof of concept.
Kamal KY, Othman MA, Kim JH, Lawler JM. Kamal KY, et al. NPJ Microgravity. 2024 Jun 11;10(1):62. doi: 10.1038/s41526-023-00320-0. NPJ Microgravity. 2024. PMID: 38862543 Free PMC article. - Glycative stress inhibits hypertrophy and impairs cell membrane integrity in overloaded mouse skeletal muscle.
Egawa T, Ogawa T, Yokokawa T, Kido K, Iyama R, Zhao H, Kurogi E, Goto K, Hayashi T. Egawa T, et al. J Cachexia Sarcopenia Muscle. 2024 Jun;15(3):883-896. doi: 10.1002/jcsm.13444. Epub 2024 Apr 4. J Cachexia Sarcopenia Muscle. 2024. PMID: 38575520 Free PMC article. - Muscle Morphology Does Not Solely Determine Knee Flexion Weakness After Anterior Cruciate Ligament Reconstruction with a Semitendinosus Tendon Graft: A Combined Experimental and Computational Modeling Study.
Kositsky A, Stenroth L, Barrett RS, Korhonen RK, Vertullo CJ, Diamond LE, Saxby DJ. Kositsky A, et al. Ann Biomed Eng. 2024 May;52(5):1313-1325. doi: 10.1007/s10439-024-03455-7. Epub 2024 Feb 29. Ann Biomed Eng. 2024. PMID: 38421479 Free PMC article. - Ex-vivo validation of spatial gain sonography for the quantification of echo intensity in fascicle-aligned ultrasound images in ten anatomical muscles in Bos taurus.
Rosahl SC, Rauschendorfer P, Arndt L, Voigtmann T, Mittag U, Rittweger J. Rosahl SC, et al. Sci Rep. 2024 Feb 15;14(1):3808. doi: 10.1038/s41598-024-53852-0. Sci Rep. 2024. PMID: 38360989 Free PMC article. - The interaction of in vivo muscle operating lengths and passive stiffness in rat hindlimbs.
Horner AM, Azizi E, Roberts TJ. Horner AM, et al. J Exp Biol. 2024 Mar 1;227(5):jeb246280. doi: 10.1242/jeb.246280. Epub 2024 Mar 11. J Exp Biol. 2024. PMID: 38353270
References
- Abmayr S, Chamberlain JS. The structure and function of dystrophin. In: Winder S, editor. The Molecular Mechanisms of Muscular Dystrophy. Georgetown: Landes Bioscience; 2006.
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. - PubMed
- Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. - PubMed
- Bloch RJ, Reed P, O’Neill A, Strong J, Williams M, Porter N, Gonzalez-Serratos H. Costameres mediate force transduction in healthy skeletal muscle and are altered in muscular dystrophies. J Muscle Res Cell Motil. 2004;25:590–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL080388/HL/NHLBI NIH HHS/United States
- P30 AG13283/AG/NIA NIH HHS/United States
- R01-HL080388/HL/NHLBI NIH HHS/United States
- P01 AG015434/AG/NIA NIH HHS/United States
- P01 AG15434/AG/NIA NIH HHS/United States
- P30 AG013283/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases