Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor - PubMed (original) (raw)
Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor
Joshua G DeKeyser et al. Toxicol Sci. 2011 Apr.
Abstract
Phthalates and other endocrine-disruptive chemicals are manufactured in large quantities for use as plasticizers and other commercial applications, resulting in ubiquitous human exposure and thus, concern regarding their toxicity. Innate defense against small molecule exposures is controlled in large part by the constitutive androstane receptor (CAR) and the pregnane X receptor (PXR). The human CAR gene undergoes multiple alternative splicing events resulting in the CAR2 and CAR3 variant receptors. Recent studies from our laboratory show that CAR2 is potently and specifically activated by di(2-ethylhexyl) phthalate (DEHP). We hypothesized that alternative splicing is a mechanism for increasing CAR's functional diversity, broadening the human receptors' repertoire of response to environmental xenobiotics. In these studies, we examine the interaction of alternatively spliced CARs and PXR with a range of suspected endocrine disruptors, including phthalates, bisphenol A (BPA), and 4-N-nonylphenol (NP). Transactivation and two-hybrid studies in COS-1 cells revealed differential selectivity of endocrine-disrupting chemicals for the variant CAR and PXR. Ex vivo studies showed DEHP and di-isononyl phthalate potently induced CYP2B6 and CYP3A4 expression in human hepatocytes. Mutation analysis of CAR2, in silico modeling, and ligand docking studies suggested that the SPTV amino acid insertion of CAR2 creates a unique ligand-binding pocket. Alternative gene splicing results in variant CAR receptors that selectively recognize phthalates and BPA. The interaction of phthalates with CAR and PXR suggests a xenobiotic response that is complex and biologically redundant.
Figures
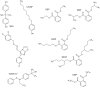
FIG. 1.
Structures of the compounds used in this study. All compounds were supplied at >98% purity and were isomerically pure, except DiNP, which was a mixture of 9-carbon isomers.
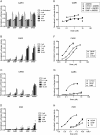
FIG. 2.
Activation of the 2B6-XREM-PBREM reporter by CAR1, CAR2, CAR3, or PXR after treatment with bisphenol A or nonylphenol. Results shown here represent a single transfection experiment, with all treatments in quadruplicate. COS-1 cells were transfected with the 3.1-RXRα expression vector, the 2B6-XREM-PBREM reporter, the pRL-CMV vector for normalization of transfection efficiency and either CMV2-CAR1, CMV2-CAR2, CMV2-CAR3, or CMVs-PXR. All treatments were for 24 h, and the data are represented as normalized luciferase values and each data point represents the mean (±SEM). *p < 0.05 compared with androstanol control; **p < 0.05 compared with DMSO control.
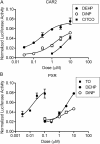
FIG. 3.
Activation of the 2B6-XREM-PBREM reporter by CAR1, CAR2, CAR3, and PXR after treatment with phthalates. Results shown here represent a single transfection experiment, with all treatments in quadruplicate. COS-1 cells were transfected with 3.1-RXRα expression vector, the 2B6-XREM-PBREM reporter, the pRL-CMV vector for normalization of transfection efficiency and either CMV2-CAR1 (A and E), CMV2-CAR2 (B and F), CMV2-CAR3 (C and G), or CMV2-PXR (D and H). Panels (A), (B), (C), and (D) show activity of various phthalates with each of the receptors. Panels (E), (F), (G), and (H) show a more complete dose-response curve comparing DEHP, DiNP, and the positive control CITCO for CAR1, CAR2, and CAR3 or TO901317 for PXR. All treatments were for 24 h, and the data are represented as normalized luciferase values and each data point represents the mean (±SEM). *p < 0.05 compared with androstanol control for CAR1 or DMSO control for CAR2, CAR3, and PXR.
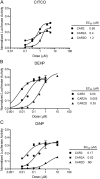
FIG. 4.
DEHP and DiNP demonstrate selectivity for CAR2 in mammalian two-hybrid assay. COS-1 cells were transfected with CAR or PXR in the pm (GAL4) vector, SRC1 in the VP16 vector, 3.1 RXRα-LBD, pFR-luciferase reporter, and pRL-CMV vector for normalization. Chemical treatments were for 24 h. Data are represented as normalized luciferase values, and each data point represents the mean (±SEM) of quadruplicate treatment wells from a representative transfection experiment.
FIG. 5.
Representative immunoblot showing expression of CYP2B6 and CYP3A4 in human hepatocytes treated with DEHP or DiNP. Human hepatocytes were treated with varying concentrations of DEHP and DiNP with DMSO, PB, and CITCO as controls for 48 h. Protein (S9 fractions) were isolated from lysed cells and subjected to immunoblot analysis with CYP2B6- or CYP3A4-specific antibodies. Actin is shown for normalization of protein loading.
FIG. 6.
Activation of the 2B6-XREM-PBREM reporter by CAR2, CAR2A, and CAR2D in response to CITCO, DEHP, and DiNP. All transfections included the 3.1-RXRα expression vector, the 2B6-XREM-PBREM reporter, the pRL-CMV vector for normalization of transfection efficiency and either CMV2-CAR2 or CMV2-CAR2A. Cells were treated for 24 h. Data are represented as normalized luciferase values, and each data point represents the mean (±SEM) of quadruplicate treatment wells from a representative transfection experiment.
FIG. 7.
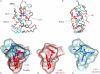
CAR2 predicted protein structure and CAR1 and CAR2 LBP models. Panels (A) and (B) show two views the Phyre homology model of the human CAR2 receptor ligand-binding domain (aqua) superimposed on the CAR1 ligand-binding domain structure (red). CITCO (blue) is shown in the LBP of CAR1. The CAR2 SPTV insertion is shown in yellow and a region adjacent to the insertion where the structure deviates from CAR1 is shown in green. Panel (C) shows an overlay of PocketFinder computer generated models of the CAR1 (red) and CAR2 (aqua) LBP with their respective ligands, CITCO (red) and DEHP (blue). Panels (D) and (E) show the individual CAR1 and CAR2 LBP.
Similar articles
- Di(2-ethylhexyl) phthalate is a highly potent agonist for the human constitutive androstane receptor splice variant CAR2.
DeKeyser JG, Stagliano MC, Auerbach SS, Prabhu KS, Jones AD, Omiecinski CJ. DeKeyser JG, et al. Mol Pharmacol. 2009 May;75(5):1005-13. doi: 10.1124/mol.108.053702. Epub 2009 Feb 11. Mol Pharmacol. 2009. PMID: 19211671 Free PMC article. - Effects of di-(2-ethylhexyl) phthalate and its metabolites on transcriptional activity via human nuclear receptors and gene expression in HepaRG cells.
Yasuda A, Murase W, Kubota A, Uramaru N, Okuda K, Hakota R, Ikeda A, Kojima H. Yasuda A, et al. Toxicol In Vitro. 2024 Dec;101:105943. doi: 10.1016/j.tiv.2024.105943. Epub 2024 Sep 26. Toxicol In Vitro. 2024. PMID: 39341470 - The nuclear pregnane X receptor regulates xenobiotic detoxification.
Kliewer SA. Kliewer SA. J Nutr. 2003 Jul;133(7 Suppl):2444S-2447S. doi: 10.1093/jn/133.7.2444S. J Nutr. 2003. PMID: 12840222 Review. - Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics.
LeCluyse EL. LeCluyse EL. Chem Biol Interact. 2001 May 16;134(3):283-9. doi: 10.1016/s0009-2797(01)00163-6. Chem Biol Interact. 2001. PMID: 11336976 Review.
Cited by
- The novel antibacterial compound walrycin A induces human PXR transcriptional activity.
Berthier A, Oger F, Gheeraert C, Boulahtouf A, Le Guével R, Balaguer P, Staels B, Salbert G, Lefebvre P. Berthier A, et al. Toxicol Sci. 2012 May;127(1):225-35. doi: 10.1093/toxsci/kfs073. Epub 2012 Feb 7. Toxicol Sci. 2012. PMID: 22314385 Free PMC article. - Oxazaphosphorine bioactivation and detoxification The role of xenobiotic receptors.
Wang D, Wang H. Wang D, et al. Acta Pharm Sin B. 2012 Apr 1;2(2):10.1016/j.apsb.2012.02.004. doi: 10.1016/j.apsb.2012.02.004. Acta Pharm Sin B. 2012. PMID: 24349963 Free PMC article. - Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture.
Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC, Coslo DM, Omiecinski CJ, Cave MC. Wahlang B, et al. Toxicol Sci. 2014 Aug 1;140(2):283-97. doi: 10.1093/toxsci/kfu083. Epub 2014 May 8. Toxicol Sci. 2014. PMID: 24812009 Free PMC article. - Mechanistic considerations for human relevance of cancer hazard of di(2-ethylhexyl) phthalate.
Rusyn I, Corton JC. Rusyn I, et al. Mutat Res. 2012 Apr-Jun;750(2):141-158. doi: 10.1016/j.mrrev.2011.12.004. Epub 2011 Dec 20. Mutat Res. 2012. PMID: 22198209 Free PMC article. Review. - Families of nuclear receptors in vertebrate models: characteristic and comparative toxicological perspective.
Zhao Y, Zhang K, Giesy JP, Hu J. Zhao Y, et al. Sci Rep. 2015 Feb 25;5:8554. doi: 10.1038/srep08554. Sci Rep. 2015. PMID: 25711679 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous