Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease - PubMed (original) (raw)
. 2011 Jan 12;31(2):700-11.
doi: 10.1523/JNEUROSCI.4152-10.2011.
Brian Halabisky, Jong W Yoo, Jinghua Yao, Jeannie Chin, Fengrong Yan, Tiffany Wu, Patricia Hamto, Nino Devidze, Gui-Qiu Yu, Jorge J Palop, Jeffrey L Noebels, Lennart Mucke
Affiliations
- PMID: 21228179
- PMCID: PMC3325794
- DOI: 10.1523/JNEUROSCI.4152-10.2011
Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease
Erik D Roberson et al. J Neurosci. 2011.
Abstract
Alzheimer's disease (AD), the most common neurodegenerative disorder, is a growing public health problem and still lacks effective treatments. Recent evidence suggests that microtubule-associated protein tau may mediate amyloid-β peptide (Aβ) toxicity by modulating the tyrosine kinase Fyn. We showed previously that tau reduction prevents, and Fyn overexpression exacerbates, cognitive deficits in human amyloid precursor protein (hAPP) transgenic mice overexpressing Aβ. However, the mechanisms by which Aβ, tau, and Fyn cooperate in AD-related pathogenesis remain to be fully elucidated. Here we examined the synaptic and network effects of this pathogenic triad. Tau reduction prevented cognitive decline induced by synergistic effects of Aβ and Fyn. Tau reduction also prevented synaptic transmission and plasticity deficits in hAPP mice. Using electroencephalography to examine network effects, we found that tau reduction prevented spontaneous epileptiform activity in multiple lines of hAPP mice. Tau reduction also reduced the severity of spontaneous and chemically induced seizures in mice overexpressing both Aβ and Fyn. To better understand these protective effects, we recorded whole-cell currents in acute hippocampal slices from hAPP mice with and without tau. hAPP mice with tau had increased spontaneous and evoked excitatory currents, reduced inhibitory currents, and NMDA receptor dysfunction. Tau reduction increased inhibitory currents and normalized excitation/inhibition balance and NMDA receptor-mediated currents in hAPP mice. Our results indicate that Aβ, tau, and Fyn jointly impair synaptic and network function and suggest that disrupting the copathogenic relationship between these factors could be of therapeutic benefit.
Figures
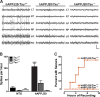
Figure 1.
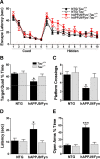
Tau reduction prevents behavioral deficits in hAPPJ9/Fyn mice. A, Morris water maze. There were no differences on the cued platform task. hAPPJ9/Fyn/Tau+/+ mice, but not hAPPJ9/Fyn/_Tau_−/− mice, were impaired on the hidden platform task (repeated-measures ANOVA, p < 0.005; on post hoc tests, only hAPPJ9/Fyn/Tau+/+ mice differed from controls; n = 9–10 mice per group; 4.5–8 months old). B–D, Morris water maze probe trial. Only hAPPJ9/Fyn/Tau+/+ mice were impaired, showing less time in the target quadrant (B), fewer platform crossings (C), and a longer latency to the first crossing of the location where the target platform had been (D). *p < 0.05 on post hoc tests. E, Elevated plus maze. hAPPJ9/Fyn/Tau+/+ mice showed abnormally high time in the open arms, but hAPPJ9/Fyn/_Tau_−/− mice did not (ANOVA, p < 0.0001; Tau × hAPPJ9/Fyn interaction, p < 0.0005; on post hoc tests, hAPPJ9/Fyn/Tau+/+ mice differed from other groups, ***p < 0.001; n = 9–10 mice per group; 3.5–7 months old).
Figure 2.
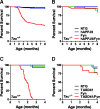
Tau reduction prevents early mortality in hAPPJ9/Fyn mice and TASD41/Fyn mice. A, B, Kaplan–Meier survival plots in NTG, hAPPJ9, Fyn, and hAPPJ9/Fyn mice on the Tau+/+ (A) or _Tau_−/− (B) background. There was high mortality in hAPPJ9/Fyn/Tau+/+ mice (log-rank test vs other Tau+/+ groups, p < 0.0001) but not in hAPPJ9/Fyn/_Tau_−/− mice (log-rank test vs hAPPJ9/Fyn/Tau+/+, p < 0.0001). C, D, Kaplan–Meier survival plots in NTG, TASD41, Fyn, and TASD41/Fyn mice on the Tau+/+ (C) or _Tau_−/− (D) background. There was high mortality in TASD41/Fyn/Tau+/+ mice (log-rank test vs other Tau+/+ groups, p < 0.0001), whereas TASD41/Fyn/_Tau_−/− mice had longer survival (log-rank test vs TASD/Fyn/Tau+/+, p < 0.0001).
Figure 3.
Tau reduction does not alter disease onset or survival in a mouse model of ALS. A, Age at onset in SOD1G93A mice, defined as the age at which weight started to decline, was not affected by tau reduction. B, Survival to end-stage disease was not altered by tau reduction.
Figure 4.
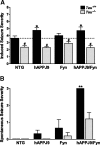
Effect of tau reduction on network excitability and spontaneous epileptiform activity in hAPP mice. A, Induced seizure severity was monitored after intraperitoneal injection of 40 mg/kg PTZ in NTG, hAPPJ9, Fyn, and hAPPJ9/Fyn mice (Tau effect, p < 0.001 by ANOVA; *p < 0.05 vs NTG and #p < 0.05 vs corresponding Tau+/+ group by post hoc testing; n = 16–20 mice per group; 5–8 months old). B, Severity of electrographically detected spontaneous seizures was scored (Tau effect, p < 0.005 by ANOVA; **p < 0.01 by post hoc tests; n = 5–6 mice in each group, median 8 h of EEG; 8–10 months old).
Figure 5.
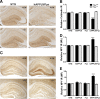
Tau reduction prevents hippocampal remodeling in hAPPJ9/Fyn mice. A, B, Calbindin levels in the molecular layer of the dentate gyrus were determined by immunohistochemistry (representative examples in A) and quantified by densitometry (B). Levels were significantly decreased in hAPPJ9/Fyn mice. This change was prevented by tau reduction (Tau × hAPPJ9/Fyn interaction, p < 0.001; ***p < 0.0001 by post hoc testing; n = 38–43 mice for Tau+/+ groups and 11–12 mice for _Tau_−/− groups; 6–9 months old). C–E, NPY levels were determined by immunohistochemistry (representative examples in C). Immunoreactivity in the molecular layer (D) and mossy fibers (E) was quantified by densitometry. Levels were not changed in hAPPJ9 or Fyn mice but were significantly increased in hAPPJ9/Fyn mice; the increase was prevented by tau reduction (Tau × hAPPJ9/Fyn interaction, p < 0.001; ***p < 0.0001 by post hoc testing; n = 43–53 mice for Tau+/+ groups and 18–20 mice for _Tau_−/− groups; 6–9 months old).
Figure 6.
Tau reduction prevents spontaneous epileptiform activity in hAPPJ20 mice. A, EEG tracings from hAPPJ20 mice with different levels of tau expression had a high frequency of generalized epileptiform spikes in the presence of normal tau levels but markedly fewer spikes in the presence of reduced tau levels. L, Left; R, right; T, temporal; P, parietal; O, occipital. Calibration bars: 2 s, 0.6 mV. B, Quantification of spontaneous epileptiform spiking in NTG and hAPPJ20 mice with different tau levels. Tau reduction blocked Aβ-induced spiking in a dose-dependent manner (hAPP × tau interaction, p < 0.001 by ANOVA; ***p < 0.001 vs all other groups by post hoc test; n = 20–32 h of EEG from 2–4 mice for each of NTG groups, 48–77 h from 5–10 mice for each of the hAPPJ20 groups; 7–14 months old). C, Kaplan–Meier analysis showing the duration of recording until an electrographic seizure was captured in hAPPJ20 mice with different tau levels. No seizures were observed in hAPPJ20/Tau+/− or hAPPJ20/_Tau_−/− mice (log-rank test, p = 0.05).
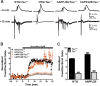
Figure 7.
Tau reduction prevents epileptiform bursting in acute hippocampal slices from NTG and hAPPJ20 mice. Bicuculline (10 μ
m
) was superfused into the bath during recording in area CA1, and resulting epileptiform discharges were quantified by CBI. A, Representative traces 5 min before and 15 min after bicuculline. Note that traces after bicuculline are plotted on a longer timescale to accommodate the prolonged response. B, Time course. C, Average CBI during minutes 15–25. Slices from hAPPJ20/Tau+/+ mice had larger epileptiform bursts than slices from NTG/Tau+/+ mice, and tau reduction markedly reduced bursting in both NTG and hAPPJ20 slices (hAPP × tau interaction, p < 0.002 by two-way ANOVA; ***p < 0.001 vs all other groups; ###p < 0.001 vs Tau+/+ groups; NTG/_Tau_−/− and hAPPJ20/_Tau_−/− were not significantly different; n = 6–8 slices from 3–4 mice per genotype).
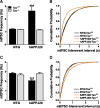
Figure 8.
Tau reduction prevents abnormalities in miniature postsynaptic potentials in dentate granule cells of hAPPJ20 mice. Whole-cell recordings were made from acute hippocampal slices. A and C show mean frequency; B and D show cumulative probability plots for interevent intervals. A, B, mIPSC frequency was increased in hAPPJ20 granule cells, and the change was blocked by tau reduction (***p < 0.001 vs NTG/Tau+/+ by Kolmogorov–Smirnoff test). C, D, mEPSC frequency was reduced in hAPPJ20 granule cells, and the change was blocked by tau reduction (***p < 0.001 vs NTG/Tau+/+ by Kolmogorov–Smirnoff test). For both mIPSC and mEPSCs, n = 2000–2400 events from 10–12 cells (200 events from each) in 4 mice per genotype.
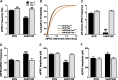
Figure 9.
Tau reduction prevents excitation–inhibition imbalance in dentate granule cells of hAPPJ20 mice. Whole-cell recordings were made from acute hippocampal slices without pharmacological receptor blockers. A, B, sIPSC frequency was lower in hAPPJ20 granule cells and higher in Tau_−/− groups than in NTG/Tau+/+ controls (***p < 0.001 and **p < 0.01 vs NTG/Tau+/+ by Kolmogorov–Smirnoff test; n = 2800–3000 events from 14–15 cells, 200 events from each, in 4 mice per genotype). C, Frequency of action potential-driven IPSCs. For each cell from which both sIPSC and mIPSC frequency data were obtained, we subtracted the frequency of mIPSCs (recorded in the presence of TTX; see Fig. 8_A) from the frequency of sIPSCs; the difference reflects IPSCs driven by action potentials. hAPPJ20/Tau+/+ cells had almost no action potential-driven IPSCs, because the sIPSC frequency was essentially the same as the mIPSC frequency (hAPP × tau interaction, p < 0.001 by two-way ANOVA; ***p < 0.001 vs all other groups by post hoc test; n = 10–12 cells from 4 mice per genotype). D, hAPPJ20/Tau+/+ cells had an increased ratio of sEPSC to sIPSC frequency, and this abnormality was prevented by tau reduction (hAPP × tau interaction, p < 0.05 by two-way ANOVA; ***p < 0.001 vs all other groups by post hoc test; n = 14–15 cells from 4 mice per genotype). E, Amplitude of IPSCs onto granule cells evoked by stimulation in the molecular layer was reduced in hAPPJ20/Tau+/+ mice but not in hAPPJ20/_Tau_−/− mice (hAPP × tau interaction, p < 0.002 by two-way ANOVA; ***p < 0.001 vs all other groups by post hoc test; n = 10 cells from 4 mice per genotype). F, Amplitude of evoked EPSCs onto granule cells was mildly increased in hAPPJ20/Tau+/+ mice but not in hAPPJ20/_Tau_−/− mice (hAPP × tau interaction, p = 0.14; hAPP effect, p < 0.05 by two-way ANOVA; *p < 0.05 vs NTG/_Tau_−/− by post hoc test; n = 10 cells from 4 mice per genotype).
Figure 10.
Tau reduction prevents abnormalities in synaptic transmission and plasticity in hAPPJ20 mice. Field (A, B, D) and whole-cell (C) recordings were made from acute hippocampal slices. A, Paired-pulse ratio in the medial perforant pathway was reduced in hAPPJ20/Tau+/+ mice but normal in hAPPJ20/_Tau_−/− mice (hAPP × tau interaction, p < 0.01 by two-way ANOVA; **p < 0.01 vs all other groups by post hoc tests; n = 8 slices from 3–4 mice per genotype.) B, Theta burst stimulation-induced LTP at the medial perforant path synapse was impaired in hAPPJ20/Tau+/+ mice, and this deficit was blocked by tau reduction (repeated-measures ANOVA on data from minutes 51–60, p < 0.01; n = 10–12 slices from 3–4 mice per genotype). C, Evoked NMDA and AMPA currents were recorded by whole-cell patch clamp on dentate granule cells. NMDA/AMPA ratios were depressed in hAPPJ20/Tau+/+ mice, and this deficit was blocked by tau reduction (hAPP × tau interaction, p < 0.001 by two-way ANOVA; ***p < 0.001 vs all other groups by post hoc tests; n = 15–18 cells from 4 mice per genotype). D, Synaptic strength in area CA1 was significantly decreased in hAPPJ20/Tau+/+ mice (repeated-measures ANOVA, p < 0.01) but normal in hAPPJ20/_Tau_−/− mice (n = 10–12 slices from 3–4 mice per genotype).
Similar articles
- Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease.
Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Chin J, et al. J Neurosci. 2005 Oct 19;25(42):9694-703. doi: 10.1523/JNEUROSCI.2980-05.2005. J Neurosci. 2005. PMID: 16237174 Free PMC article. - Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice.
Chin J, Palop JJ, Yu GQ, Kojima N, Masliah E, Mucke L. Chin J, et al. J Neurosci. 2004 May 12;24(19):4692-7. doi: 10.1523/JNEUROSCI.0277-04.2004. J Neurosci. 2004. PMID: 15140940 Free PMC article. - The complex PrP(c)-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer's disease.
Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, Aguzzi A, Lesné SE. Larson M, et al. J Neurosci. 2012 Nov 21;32(47):16857-71a. doi: 10.1523/JNEUROSCI.1858-12.2012. J Neurosci. 2012. PMID: 23175838 Free PMC article. - Epilepsy and cognitive impairments in Alzheimer disease.
Palop JJ, Mucke L. Palop JJ, et al. Arch Neurol. 2009 Apr;66(4):435-40. doi: 10.1001/archneurol.2009.15. Epub 2009 Feb 9. Arch Neurol. 2009. PMID: 19204149 Free PMC article. Review. - Tau acts as a mediator for Alzheimer's disease-related synaptic deficits.
Liao D, Miller EC, Teravskis PJ. Liao D, et al. Eur J Neurosci. 2014 Apr;39(7):1202-13. doi: 10.1111/ejn.12504. Eur J Neurosci. 2014. PMID: 24712999 Free PMC article. Review.
Cited by
- Fragile X mental retardation protein: from autism to neurodegenerative disease.
Wang H. Wang H. Front Cell Neurosci. 2015 Feb 12;9:43. doi: 10.3389/fncel.2015.00043. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25729352 Free PMC article. No abstract available. - Tau Accumulation, Altered Phosphorylation, and Missorting Promote Neurodegeneration in Glaucoma.
Chiasseu M, Cueva Vargas JL, Destroismaisons L, Vande Velde C, Leclerc N, Di Polo A. Chiasseu M, et al. J Neurosci. 2016 May 25;36(21):5785-98. doi: 10.1523/JNEUROSCI.3986-15.2016. J Neurosci. 2016. PMID: 27225768 Free PMC article. - Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice.
Cheng JS, Craft R, Yu GQ, Ho K, Wang X, Mohan G, Mangnitsky S, Ponnusamy R, Mucke L. Cheng JS, et al. PLoS One. 2014 Dec 31;9(12):e115765. doi: 10.1371/journal.pone.0115765. eCollection 2014. PLoS One. 2014. PMID: 25551452 Free PMC article. - A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease.
Davis EJ, Broestl L, Abdulai-Saiku S, Worden K, Bonham LW, Miñones-Moyano E, Moreno AJ, Wang D, Chang K, Williams G, Garay BI, Lobach I, Devidze N, Kim D, Anderson-Bergman C, Yu GQ, White CC, Harris JA, Miller BL, Bennett DA, Arnold AP, De Jager PL, Palop JJ, Panning B, Yokoyama JS, Mucke L, Dubal DB. Davis EJ, et al. Sci Transl Med. 2020 Aug 26;12(558):eaaz5677. doi: 10.1126/scitranslmed.aaz5677. Sci Transl Med. 2020. PMID: 32848093 Free PMC article. - Neuronal Network Excitability in Alzheimer's Disease: The Puzzle of Similar versus Divergent Roles of Amyloid β and Tau.
Kazim SF, Seo JH, Bianchi R, Larson CS, Sharma A, Wong RKS, Gorbachev KY, Pereira AC. Kazim SF, et al. eNeuro. 2021 Apr 23;8(2):ENEURO.0418-20.2020. doi: 10.1523/ENEURO.0418-20.2020. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33741601 Free PMC article. Review.
References
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. - PubMed
- Cain DP, Grant SG, Saucier D, Hargreaves EL, Kandel ER. Fyn tyrosine kinase is required for normal amygdala kindling. Epilepsy Res. 1995;22:107–114. - PubMed
- Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet. 1997;6:1951–1959. - PubMed
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS29709/NS/NINDS NIH HHS/United States
- R01 NS041787-11/NS/NINDS NIH HHS/United States
- NS041787/NS/NINDS NIH HHS/United States
- R01 NS041787/NS/NINDS NIH HHS/United States
- RR018928/RR/NCRR NIH HHS/United States
- NS054811/NS/NINDS NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- R01 NS029709/NS/NINDS NIH HHS/United States
- P01 AG022074-09/AG/NIA NIH HHS/United States
- C06 RR018928/RR/NCRR NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- K08 NS054811/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous