HDAC3 is a critical negative regulator of long-term memory formation - PubMed (original) (raw)
HDAC3 is a critical negative regulator of long-term memory formation
Susan C McQuown et al. J Neurosci. 2011.
Abstract
Gene expression is dynamically regulated by chromatin modifications on histone tails, such as acetylation. In general, histone acetylation promotes transcription, whereas histone deacetylation negatively regulates transcription. The interplay between histone acetyltranserases and histone deacetylases (HDACs) is pivotal for the regulation of gene expression required for long-term memory processes. Currently, very little is known about the role of individual HDACs in learning and memory. We examined the role of HDAC3 in long-term memory using a combined genetic and pharmacologic approach. We used HDAC3-FLOX genetically modified mice in combination with adeno-associated virus-expressing Cre recombinase to generate focal homozygous deletions of Hdac3 in area CA1 of the dorsal hippocampus. To complement this approach, we also used a selective inhibitor of HDAC3, RGFP136 [N-(6-(2-amino-4-fluorophenylamino)-6-oxohexyl)-4-methylbenzamide]. Immunohistochemistry showed that focal deletion or intrahippocampal delivery of RGFP136 resulted in increased histone acetylation. Both the focal deletion of HDAC3 as well as HDAC3 inhibition via RGFP136 significantly enhanced long-term memory in a persistent manner. Next we examined expression of genes implicated in long-term memory from dorsal hippocampal punches using quantitative reverse transcription-PCR. Expression of nuclear receptor subfamily 4 group A, member 2 (Nr4a2) and c-fos was significantly increased in the hippocampus of HDAC3-FLOX mice compared with wild-type controls. Memory enhancements observed in HDAC3-FLOX mice were abolished by intrahippocampal delivery of Nr4a2 small interfering RNA, suggesting a mechanism by which HDAC3 negatively regulates memory formation. Together, these findings demonstrate a critical role for HDAC3 in the molecular mechanisms underlying long-term memory formation.
Figures
Figure 1.
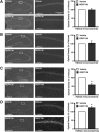
Intrahippocampal AAV2/1–Cre infusion in HDAC3flox/flox mice results in a complete, focal deletion of HDAC3 that correlates with increased histone acetylation. Images are 4×, except the right panels, which are 20× magnifications of the regions boxed in white. Histograms depict quantification of optical density as a percentage of wild type. A, Representative images showing DAPI labeling and HDAC3 immunoreactivity in hippocampi of AAV2/1–Cre infused HDAC3+/+ and HDAC3flox/flox mice. HDAC3 labeling is found throughout CA1, CA3, and the dentate gyrus, and no immunoreactivity is found in the AAV2/1–Cre infusion site of HDAC3flox/flox mice. *p < 0.05. B, Representative images showing HDAC2 immunoreactivity in hippocampus is unchanged in AAV2/1–Cre-infused HDAC3flox/flox mice. C, However, HDAC4 immunoreactivity is decreased in the region of the HDAC3 deletion. *p < 0.05. D, Furthermore, acetylation at H4K8 is increased specifically in the AAV2/1–Cre infusion site of HDAC3flox/flox mice. *p < 0.05.
Figure 2.
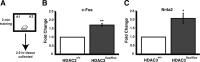
c-fos and Nr4a2 expression are increased in the area of focal homozygous deletion of Hdac3 in HDAC3flox/flox mice. A, Mice received subthreshold training (3 min) in an environment with two identical objects. B, Two hours after training, quantitative RT-PCR shows that c-fos expression is significantly increased in the dorsal hippocampus of HDAC3flox/flox mice compared with wild-type littermates (n = 3 per group; **p < 0.003). C, In addition, training induced greater Nr4a2 expression in the dorsal hippocampus of HDAC3flox/flox mice compared with wild-type littermates (n = 3 per group; *p < 0.02).
Figure 3.
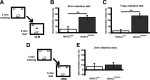
Focal homozygous gene deletion of Hdac3 in the dorsal hippocampus leads to enhanced memory for object location (OLM), which persists at least 7 d but not for object recognition (ORM). A, Mice received subthreshold training (3 min) in an environment with two identical objects and received a retention test 24 h or 7 d later in which one object is moved to a new location. Schematic describes methods for B and C. B, HDAC3flox/flox mice exhibited significant long-term memory for object location 24 h after subthreshold training (n = 8 per group; **p < 0.005). C, In a different set of mice, the persistence of this enhanced memory was examined. HDAC3flox/flox mice displayed a significant preference for the novel object location compared with HDAC3+/+ mice during a 7 d retention test (n = 9 per group; **p < 0.001). D, Mice received subthreshold training (3 min) in an environment with two identical objects and received a retention test 24 h later in which one object is replaced with a novel one (ORM). Schematic describes methods for E. E, Neither HDAC3+/+ or HDAC3flox/flox mice exhibited significant preference for the novel object (n = 8 per group).
Figure 4.
Loss of HDAC3/NCoR interaction enhances long-term OLM and ORM formation but has no effect on short-term memory. A, Mice received subthreshold training (3 min) in an environment with two identical objects and received a retention test 90 min (B) or 24 h (C) later in which one object is moved to a new location. B, Subthreshold training did not result in significant short-term memory by either genotype when tested 90 min later (n = 11–13 per group). C, However, DADm mice showed a significant preference for the novel object location 24 h after training compared with wild types (n = 9–10 per group; **p < 0.005). D, Mice received subthreshold training (3 min) in an environment with two identical objects and received a retention test 24 h later in which one object is replaced with a novel one (ORM). Schematic describes methods for E. E, DADm mice showed a significant preference for the novel object itself 24 h after training compared with wild types (n = 12–18 per group; *p < 0.05).
Figure 5.
Intrahippocampal RGFP136 infusions increases histone acetylation. Images on left are 4×, and 20× magnifications of the regions boxed in white are on the right. Histograms depict quantification of optical density as a percentage of vehicle. A, HDAC3 immunoreactivity is unaltered in area of infusion 2 h after RGFP136 treatment compared with vehicle. B, Representative images show HDAC2 immunoreactivity in dorsal hippocampus is also unchanged by drug treatment. C, However, HDAC4 nuclear immunoreactivity is decreased in the region of the RGFP136 infusion. *p < 0.05. D, Furthermore, acetylation at H4K8 is increased in RGFP136-infused mice compared with those treated with vehicle. *p < 0.05.
Figure 6.
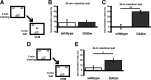
The HDAC inhibitor RGFP136 enhances long-term memory for ORM and OLM. A, Mice received subthreshold training (3 min) in an environment with two identical objects immediately followed by subcutaneous injection of RGFP136 and received a retention test 24 h (B) or 7 d (C) later in which one object is replaced with a novel one. B, Mice treated with the 30 mg/kg dose exhibited a significant preference for the novel object than vehicle-treated controls, whereas 150 mg/kg treatment resulted in memory no different from vehicle (n = 7–9 per group; two-way ANOVA, **p < 0.01). C, In a different set of mice, the persistence of this enhanced memory was examined. Mice receiving subcutaneous injection of RGFP136 (30 mg/kg) exhibited significantly increased exploration of the novel object compared with vehicle-treated mice during a 7 d retention test (n = 9–10 per group; **p < 0.01). D, Mice received subthreshold training (3 min) in an environment with two identical objects immediately followed by a subcutaneous injection of RGFP136 (30 mg/kg) or vehicle and received a retention test 90 min (E) or 24 h (F) later in which one object is moved to a new location. E, Subthreshold training did not result in significant short-term memory after RGFP136 (30 mg/kg) or vehicle injections when tested 90 min later. F, Mice treated with the 30 mg/kg RGFP136 exhibited significant preference for the object in the novel location compared with vehicle-treated controls (n = 9–10 per group; **p < 0.001). G, Mice received subthreshold training (3 min) in an environment with two identical objects followed immediately by intrahippocampal infusion of RGFP136 and received a retention test 24 h (H) or 7 d (I) later in which one object is moved to a new location. H, Intrahippocampal RGFP136 treatment led to significant preference for the novel object location 24 h after subthreshold training (n = 7 per group; **p < 0.01). I, In a different set of mice, the persistence of this enhanced memory was examined. Mice receiving intrahippocampal RGFP136 also displayed a significant preference for the novel object location compared with vehicle-treated mice during a 7 d retention test (n = 8 per group; **p < 0.001).
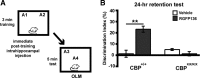
Figure 7.
The HDAC inhibitor RGFP136 requires CBP to enhance long-term memory of OLM. A, Mice received subthreshold training (3 min) in an environment with two identical objects immediately followed by intrahippocampal infusions of RGFP136 (1.25 ng/side) or vehicle (0.5 μl/side) and received a retention test 24 h later in which one object is moved to a new location. B, Wild-type CBP+/+ mice that received intrahippocampal RGFP136 immediately after training showed significant long-term memory for the object location compared with vehicle-treated mice. CBPKIX/KIX mice showed no effect of drug treatment (n = 5–9 per group; two-way ANOVA, **p < 0.001).
Figure 8.
Nr4a2 siRNA attenuates the long-term memory enhancement observed in HDAC3flox/flox mice. A, At 48 h after infusions of Nr4a2 or RISC-free siRNA, HDAC3flox/flox and HDAC3+/+ mice received subthreshold training (3 min) in an environment with two identical objects and received a retention test 24 h later in which one object is moved to a new location. B, HDAC3flox/flox mice infused with RISC free (n = 10) exhibited significant memory for object location compared with HDAC3+/+ mice (††p = 0.001), which was blocked by Nr4a2 siRNA treatment (n = 9–10 per group; **p < 0.001). C, At 2 h after testing, quantitative RT-PCR shows that Nr4a2 siRNA treatment significantly reduced Nr4a2 expression in both HDAC3flox/flox and HDAC3+/+ mice (n = 3 per group; **p < 0.001 and *p < 0.05 vs respective RISC-free siRNA controls). HDAC3flox/flox mice also exhibited an increased induction of Nr4a2 mRNA after the long-term memory test (††p = 0.002 vs HDAC3+/+ RISC free).
Similar articles
- HDAC3 is a negative regulator of cocaine-context-associated memory formation.
Rogge GA, Singh H, Dang R, Wood MA. Rogge GA, et al. J Neurosci. 2013 Apr 10;33(15):6623-32. doi: 10.1523/JNEUROSCI.4472-12.2013. J Neurosci. 2013. PMID: 23575859 Free PMC article. - HDAC3-Mediated Repression of the Nr4a Family Contributes to Age-Related Impairments in Long-Term Memory.
Kwapis JL, Alaghband Y, López AJ, Long JM, Li X, Shu G, Bodinayake KK, Matheos DP, Rapp PR, Wood MA. Kwapis JL, et al. J Neurosci. 2019 Jun 19;39(25):4999-5009. doi: 10.1523/JNEUROSCI.2799-18.2019. Epub 2019 Apr 18. J Neurosci. 2019. PMID: 31000586 Free PMC article. - Context and Auditory Fear are Differentially Regulated by HDAC3 Activity in the Lateral and Basal Subnuclei of the Amygdala.
Kwapis JL, Alaghband Y, López AJ, White AO, Campbell RR, Dang RT, Rhee D, Tran AV, Carl AE, Matheos DP, Wood MA. Kwapis JL, et al. Neuropsychopharmacology. 2017 May;42(6):1284-1294. doi: 10.1038/npp.2016.274. Epub 2016 Dec 7. Neuropsychopharmacology. 2017. PMID: 27924874 Free PMC article. - Histone deacetylase 3 inhibitors in learning and memory processes with special emphasis on benzamides.
Amin SA, Adhikari N, Kotagiri S, Jha T, Ghosh B. Amin SA, et al. Eur J Med Chem. 2019 Mar 15;166:369-380. doi: 10.1016/j.ejmech.2019.01.077. Epub 2019 Jan 31. Eur J Med Chem. 2019. PMID: 30735902 Review. - HDAC3 and the molecular brake pad hypothesis.
McQuown SC, Wood MA. McQuown SC, et al. Neurobiol Learn Mem. 2011 Jul;96(1):27-34. doi: 10.1016/j.nlm.2011.04.005. Epub 2011 Apr 16. Neurobiol Learn Mem. 2011. PMID: 21521655 Free PMC article. Review.
Cited by
- HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner.
Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA. Malvaez M, et al. Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2647-52. doi: 10.1073/pnas.1213364110. Epub 2013 Jan 7. Proc Natl Acad Sci U S A. 2013. PMID: 23297220 Free PMC article. - The promise and perils of HDAC inhibitors in neurodegeneration.
Didonna A, Opal P. Didonna A, et al. Ann Clin Transl Neurol. 2015 Jan;2(1):79-101. doi: 10.1002/acn3.147. Epub 2014 Dec 3. Ann Clin Transl Neurol. 2015. PMID: 25642438 Free PMC article. Review. - Molecular mechanisms underlying the memory-enhancing effects of estradiol.
Frick KM. Frick KM. Horm Behav. 2015 Aug;74:4-18. doi: 10.1016/j.yhbeh.2015.05.001. Epub 2015 May 8. Horm Behav. 2015. PMID: 25960081 Free PMC article. Review. - Targeting neuronal epigenomes for brain rejuvenation.
Zocher S. Zocher S. EMBO J. 2024 Aug;43(16):3312-3326. doi: 10.1038/s44318-024-00148-8. Epub 2024 Jul 15. EMBO J. 2024. PMID: 39009672 Free PMC article. Review. - Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats.
Castino MR, Cornish JL, Clemens KJ. Castino MR, et al. PLoS One. 2015 Apr 16;10(4):e0124796. doi: 10.1371/journal.pone.0124796. eCollection 2015. PLoS One. 2015. PMID: 25880762 Free PMC article.
References
- Agalioti T, Chen G, Thanos D. Deciphering the transcriptional histone acetylation code for a human gene. Cell. 2002;111:381–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH081004/MH/NIMH NIH HHS/United States
- R01 DA025922/DA/NIDA NIH HHS/United States
- DK43806/DK/NIDDK NIH HHS/United States
- R01DA025922/DA/NIDA NIH HHS/United States
- F31 MH085494/MH/NIMH NIH HHS/United States
- R01MH081004/MH/NIMH NIH HHS/United States
- R01 DK043806/DK/NIDDK NIH HHS/United States
- R01 MH081004-05/MH/NIMH NIH HHS/United States
- F31MH85494/MH/NIMH NIH HHS/United States
- R37 DK043806-20/DK/NIDDK NIH HHS/United States
- R37 DK043806/DK/NIDDK NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous