Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma - PubMed (original) (raw)
. 2011 Apr 28;117(17):4530-41.
doi: 10.1182/blood-2010-08-303354. Epub 2011 Jan 12.
Bo Yu, Beth A Christian, Fengting Yan, Jungook Shin, Rosa Lapalombella, Erin Hertlein, Mark E Lustberg, Carl Quinion, Xiaoli Zhang, Gerard Lozanski, Natarajan Muthusamy, Mette Prætorius-Ibba, Owen A O'Connor, David M Goldenberg, John C Byrd, Kristie A Blum, Robert A Baiocchi
Affiliations
- PMID: 21228331
- PMCID: PMC3099572
- DOI: 10.1182/blood-2010-08-303354
Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma
Lapo Alinari et al. Blood. 2011.
Abstract
Mantle cell lymphoma (MCL) is an aggressive B-cell malignancy with a median survival of 3 years despite chemoimmunotherapy. Rituximab, a chimeric anti-CD20 monoclonal antibody (mAb), has shown only modest activity as single agent in MCL. The humanized mAb milatuzumab targets CD74, an integral membrane protein linked with promotion of B-cell growth and survival, and has shown preclinical activity against B-cell malignancies. Because rituximab and milatuzumab target distinct antigens and potentially signal through different pathways, we explored a preclinical combination strategy in MCL. Treatment of MCL cell lines and primary tumor cells with immobilized milatuzumab and rituximab resulted in rapid cell death, radical oxygen species generation, and loss of mitochondrial membrane potential. Cytoskeletal distrupting agents significantly reduced formation of CD20/CD74 aggregates, cell adhesion, and cell death, highlighting the importance of actin microfilaments in rituximab/milatuzumab-mediated cell death. Cell death was independent of caspase activation, Bcl-2 family proteins or modulation of autophagy. Maximal inhibition of p65 nuclear translocation was observed with combination treatment, indicating disruption of the NF-κB pathway. Significant in vivo therapeutic activity of combination rituximab and milatuzumab was demonstrated in a preclinical model of MCL. These data support clinical evaluation of combination milatuzumab and rituximab therapy in MCL.
Figures
Figure 1
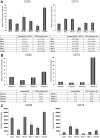
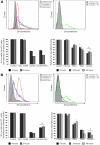
CD20 and CD74 expression on MCL cell lines and primary MCL tumor cells. (A-B) CD20 and CD74 MFI and the percentage of positive cells based on comparison to isotype control in 6 MCL cell lines (A) and primary cells from 4 different MCL patients (B). (C) The number of CD20 and CD74 molecules per cell in 6 MCL cell lines.
Figure 2
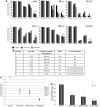
Immobilized rituximab and milatuzumab treatment-induced cell death in MCL cells. The 6 MCL cell lines (A), patient characteristics (B), and primary cells from 7 patients (C-D) were treated with rituximab (10 μg/mL) and/or milatuzumab (5 μg/mL), in the presence of a cross-linking antibody. Cell death was determined by annexin V–PI staining and flow cytometry at 8, 24, and 48 hours for the 6 cell lines and at 24 hours for MCL primary cells. Data are shown as the percentage of annexin V−PI cells (live cells) and are normalized to untreated control. Individual patient responses (C) and representative histograms summarizing patient responses (D) are shown. Combination treatment resulted in statistically significant enhanced induction of MCL cell death compared with either agent alone (P < .01).
Figure 3
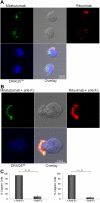
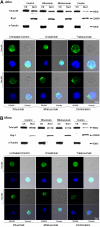
CD20 and CD74 labeling by specific fluorescent antibodies. Binding and internalization of CD20 and CD74 in Jeko cells were examined by laser scanning confocal microscopy. Jeko cells were incubated with rhodamine-conjugated rituximab (red) and Alexa Fluor 488-conjugated milatuzumab (green) in the absence (A) or presence (B) of a cross-linking antibody for 2 hours at 37°C. DRAQ5 was used for nuclear staining (blue). Cross-linked milatuzumab forms large aggregates and colocalizes with cross-linked rituximab on the surface of MCL cells. (C) Representative histograms summarizing the percentage of capped cells in the presence or absence of a cross-linking antibody are shown (Jeko cells left and Mino cells right). At least 100 cell/conditions were counted for each of the 3 independent experiments. Anti–Fc indicates anti–Fc cross-linking antibody.
Figure 4
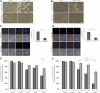
Cell-cell interactions and cytoskeleton organization are involved in MCL cell death evoked by immobilized rituximab and milatuzumab. Jeko (A) and Mino (B) cells were treated with dimethylsulfoxide (DMSO) or the actin polymerization inhibitor, latrunculin B (10μM), for 45 minutes before the addition of rituximab and milatuzumab, in the presence of a cross-linking antibody. Cell aggregation was assessed 4 hours later by light microscopy. Pretreatment with latrunculin B significantly reduced cell aggregation. Jeko (C) and Mino (D) cells were treated with DMSO or an actin polymerization inhibitor (latrunculin B), for 45 minutes before the addition of rituximab and milatuzumab in the presence of a rhodamine-conjugated cross-linking antibody. The formation of aggregates and caps are shown on Jeko in panel C (left) and Mino in panel D (left) cell surface was evaluated 4 hours later by confocal microscopy. Representative histograms summarizing the percentage of capped cells in the presence or absence of latrunculin B are shown in panel C (Jeko right) and panel D (Mino right). At least 100 cell/conditions were counted for each of the 3 independent experiments. Pretreatment with latrunculin B significantly (P < .01) reduced the capping and colocalization of CD74 and CD20 antigens. Cell death evaluation of Jeko (E) and Mino (F) cells treated with DMSO or latrunculin B, for 45 minutes before the addition of rituximab and milatuzumab in the presence of a cross-linking antibody. Cell death was determined 4 hours later by AnnexinV–PI and flow cytometry. Pretreatment with lactrunculin B resulted in statistically significant reduced cell death induced by the combination treatment (P < .01).
Figure 5
Rituximab and milatuzumab cytotoxicity is partially dependent on ROS generation and loss of ΔΨm. (A-B) Jeko in panel A (top left) and Mino in panel B (top left) cells were treated with rituximab and milatuzumab in the presence of a cross-linking antibody for the indicated time points. ROS generation was determined by flow cytometric analysis using dihydroethidine dye. ROS generation is indicated by right shift of the dihydroethidine curves. Hydrogen peroxide was used as positive control. Top right panel shows pretreatment of MCL cells with the nonspecific ROS scavenger NAC (10mM) and inhibition of ROS generation induced by antibody treatment. For rescue experiments, Jeko (bottom left panel) and Mino (bottom left panel) cells were incubated with NAC for 4 and 8 hours in the absence or presence of the antibodies, and cell viability was determined by annexin V–PI staining and flow cytometry. Bottom right panel shows ΔΨm changes in Jeko and Mino cells treated with rituxmab, milatuzumab, or the combination of both in the presence of a cross-linking antibody, for 4, 8 and 24 hours. The ΔΨm changes were quantified by flow cytometry determination using JC-1. Data are shown as the percentage of treated cells with intact mitochondria relative to untreated cells at the same time points.
Figure 6
Rituximab and milatuzumab combination treatment inhibited nuclear total p65 translocation in MCL cell lines. Top panels show nuclear and cytosolic protein fractions collected at 4 hours prepared from Jeko (A) and Mino (B) cell lines and subjected to immunoblot analysis for p65, α-tubulin (cytoplasmic control), and Brg-1 (nuclear control). Bottom panels show confocal microscopic analysis of intracellular localization of total p65 (green) in Jeko (A) and Mino (B) cells. MCL cells were treated with cross-linking antibody, trastuzumab, rituximab, milatuzumab, and the combination of rituximab and milatuzumab in the presence of a cross-linking antibody. After 4 hours, cells were collected and prepared for confocal microscopy analysis. DRAQ5 was used for nuclear staining.
Figure 7
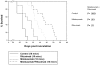
Evaluation of in vivo therapeutic activity of rituximab and milatuzumab in the preclinical MCL model. SCID mice (in groups of 10) were injected intravenously with 40 × 106 Jeko cells and observed daily for signs of tumor burden. The mAbs (trastuzumab 15 mg/kg, rituximab 15 mg/kg, milatuzumab 15 mg/kg, or the combination of rituximab and milatuzumab) were given every 3 days via intraperitoneal.injection, starting at day 15 after engraftment. The mean survival for rituximab- and milatuzumab-treated mice was 44.5 days (95% CI, 39%-51%), compared with 33.5 days for the milatuzumab-treated mice (95% CI, 28%-36%), and 38 days for the rituximab-treated mice (95% CI, 36%-42%).
Similar articles
- FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death.
Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C, Towns WH, Chen CS, Goldenberg DM, Blum KA, Byrd JC, Muthusamy N, Praetorius-Ibba M, Baiocchi RA. Alinari L, et al. Blood. 2011 Dec 22;118(26):6893-903. doi: 10.1182/blood-2011-06-363879. Epub 2011 Oct 31. Blood. 2011. PMID: 22042694 Free PMC article. - Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas.
Gupta P, Goldenberg DM, Rossi EA, Cardillo TM, Byrd JC, Muthusamy N, Furman RR, Chang CH. Gupta P, et al. Blood. 2012 Apr 19;119(16):3767-78. doi: 10.1182/blood-2011-09-381988. Epub 2012 Jan 23. Blood. 2012. PMID: 22271448 - Novel targeted therapies for mantle cell lymphoma.
Alinari L, Christian B, Baiocchi RA. Alinari L, et al. Oncotarget. 2012 Feb;3(2):203-11. doi: 10.18632/oncotarget.426. Oncotarget. 2012. PMID: 22361516 Free PMC article. - Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.
Plosker GL, Figgitt DP. Plosker GL, et al. Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review. - Milatuzumab - a promising new immunotherapeutic agent.
Berkova Z, Tao RH, Samaniego F. Berkova Z, et al. Expert Opin Investig Drugs. 2010 Jan;19(1):141-9. doi: 10.1517/13543780903463854. Expert Opin Investig Drugs. 2010. PMID: 19968579 Review.
Cited by
- Antibody therapy of pediatric B-cell lymphoma.
Meyer-Wentrup F, de Zwart V, Bierings M. Meyer-Wentrup F, et al. Front Oncol. 2013 Apr 2;3:68. doi: 10.3389/fonc.2013.00068. eCollection 2013. Front Oncol. 2013. PMID: 23565504 Free PMC article. - Mantle cell lymphoma in the era of precision medicine-diagnosis, biomarkers and therapeutic agents.
Inamdar AA, Goy A, Ayoub NM, Attia C, Oton L, Taruvai V, Costales M, Lin YT, Pecora A, Suh KS. Inamdar AA, et al. Oncotarget. 2016 Jul 26;7(30):48692-48731. doi: 10.18632/oncotarget.8961. Oncotarget. 2016. PMID: 27119356 Free PMC article. Review. - Aberrant Expression of and Cell Death Induction by Engagement of the MHC-II Chaperone CD74 in Anaplastic Large Cell Lymphoma (ALCL).
Wurster KD, Costanza M, Kreher S, Glaser S, Lamprecht B, Schleussner N, Anagnostopoulos I, Hummel M, Jöhrens K, Stein H, Molina A, Diepstra A, Gillissen B, Köchert K, Siebert R, Merkel O, Kenner L, Janz M, Mathas S. Wurster KD, et al. Cancers (Basel). 2021 Oct 7;13(19):5012. doi: 10.3390/cancers13195012. Cancers (Basel). 2021. PMID: 34638496 Free PMC article. - Target Therapy in Hematological Malignances: New Monoclonal Antibodies.
Podhorecka M, Markowicz J, Szymczyk A, Pawlowski J. Podhorecka M, et al. Int Sch Res Notices. 2014 Oct 29;2014:701493. doi: 10.1155/2014/701493. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27433507 Free PMC article. Review. - The Epstein-Barr Virus Lytic Protein BZLF1 as a Candidate Target Antigen for Vaccine Development.
Hartlage AS, Liu T, Patton JT, Garman SL, Zhang X, Kurt H, Lozanski G, Lustberg ME, Caligiuri MA, Baiocchi RA. Hartlage AS, et al. Cancer Immunol Res. 2015 Jul;3(7):787-94. doi: 10.1158/2326-6066.CIR-14-0242. Epub 2015 Mar 3. Cancer Immunol Res. 2015. PMID: 25735952 Free PMC article.
References
- Williams ME, Densmore JJ. Biology and therapy of mantle cell lymphoma. Curr Opin Oncol. 2005;17(5):425–431. - PubMed
- Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171(1):88–95. - PubMed
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–1213. - PubMed
- Zelenetz AD. Mantle cell lymphoma: an update on management. Ann Oncol. 2006;17(suppl 4):S12–S14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources