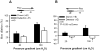H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles - PubMed (original) (raw)
H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles
Yanping Liu et al. Circ Res. 2011.
Abstract
Rationale: Endothelial derived hydrogen peroxide (H(2)O(2)) is a necessary component of the pathway regulating flow-mediated dilation (FMD) in human coronary arterioles (HCAs). However, H(2)O(2) has never been shown to be the endothelium-dependent transferrable hyperpolarization factor (EDHF) in response to shear stress.
Objective: We examined the hypothesis that H(2)O(2) serves as the EDHF in HCAs to shear stress.
Methods and results: Two HCAs were cannulated in series (a donor intact vessel upstream and endothelium-denuded detector vessel downstream). Diameter changes to flow were examined in the absence and presence of polyethylene glycol catalase (PEG-CAT). The open state probability of large conductance Ca(2+)-activated K(+) (BK(Ca)) channels in smooth muscle cells downstream from the perfusate from an endothelium-intact arteriole was examined by patch clamping. In some experiments, a cyanogen bromide-activated resin column bound with CAT was used to remove H(2)O(2) from the donor vessel. When flow proceeds from donor to detector, both vessels dilate (donor:68±7%; detector: 45±11%). With flow in the opposite direction, only the donor vessel dilates. PEG-CAT contacting only the detector vessel blocked FMD in that vessel (6±4%) but not in donor vessel (61±13%). Paxilline inhibited dilation of endothelium-denuded HCAs to H(2)O(2). Effluent from donor vessels elicited K(+) channel opening in an iberiotoxin- or PEG-CAT-sensitive fashion in cell-attached patches but had little effect on channel opening on inside-out patches. Vasodilation of detector vessels was diminished when exposed to effluent from CAT-column.
Conclusions: Flow induced endothelial production of H(2)O(2), which acts as the transferrable EDHF activating BK(Ca) channels on the smooth muscle cells.
Figures
Figure 1
Two HCAs were connected in series with donor (with endothelium, +E) upstream and detector vessel (without endothelium, -E) downstream. A. When flow proceeds from donor to detector, both donor and detector vessel dilated. B. With flow was reversed, only the donor dilated, but not detector vessels.
Figure 2
Effect of PEG-CAT (500 U/ml) on FMD in donor vessels (A) and detector vessels (B). When PEG-CAT was placed in detector vessel chamber (cross bar), FMD was inhibited in detector vessels, but was reserved in donor vessels. When PEG-CAT was placed in the perfusate traversing both vessels (gray bar), FMD was eliminated in both donor and detector vessels.
Figure 3
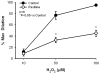
Effect of paxilline on H2O2 induce dilation in human coronary arterioles. H2O2 elicited dose-dependent vasodilation, which was significantly inhibited by 100 nM paxilline.
Figure 4
Effect of effluent from donor vessel on K+ channel activity in cell-attached patches of coronary smooth muscle cells. As indicated in sample traces (A) and summarized data (B), effluent from donor vessel greatly enhanced open state probability in cell-attached patches of smooth muscle cells. The enhancement was blocked by 100 nmol/L iberiotoxin.
Figure 5
Effect of PEG-CAT on K+ channel opening induced by effluent from donor vessel in cell-attached patches of coronary smooth muscle cells. The increased opening of K+ channels by effluent was abolished in the presence of PEG-CAT as illustrated in sample traces (A) and summarized data (B).
Figure 6
Effect of effluent from donor vessel on K+ activity in inside-out patches of coronary smooth muscle cells. K+ channel activity was significantly reduced in response to vessel effluent after elimination of intracellular components. A. Sample traces. B. Data summarized from 6 experiments.
Figure 7
A. A significant reduction in H2O2 concentration was observed in effluent from CAT-column compared to effluent from CF-column. B. Sample images comparing fluorescence intensity of DCFH representing the production of H2O2. DCFH fluorescence intensities were increased in HCA exposed to effluent from donor vessel. Fluorescence intensities were greatly reduced in HCA incubated with effluent passing through the CAT-column. C. Summary of fluorescence intensities (normalized to control) in 4 arteries of each group. Donor effluent-induced increases in DCFH fluorescence were reduced in vessels exposed to effluent from CAT-column.
Figure 8
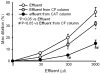
Comparison of dilator response of detector vessels to effluent from donor vessel, CF-column or CAT-column. Detector vessel dilated to effluent from donor vessels or CF-column in as volume of donor effluent increase. The dilator response was significantly reduced in detector vessels incubated in effluent from CAT-column.
Similar articles
- H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation.
Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. Zhang DX, et al. Circ Res. 2012 Feb 3;110(3):471-80. doi: 10.1161/CIRCRESAHA.111.258871. Epub 2011 Dec 8. Circ Res. 2012. PMID: 22158710 Free PMC article. - Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles.
Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Miura H, et al. Circ Res. 2003 Feb 7;92(2):e31-40. doi: 10.1161/01.res.0000054200.44505.ab. Circ Res. 2003. PMID: 12574154 - Exercise training-enhanced, endothelium-dependent dilation mediated by altered regulation of BK(Ca) channels in collateral-dependent porcine coronary arterioles.
Xie W, Parker JL, Heaps CL. Xie W, et al. Microcirculation. 2013 Feb;20(2):170-82. doi: 10.1111/micc.12016. Microcirculation. 2013. PMID: 23002811 Free PMC article. - Endothelium-derived hyperpolarising factors and associated pathways: a synopsis.
Edwards G, Félétou M, Weston AH. Edwards G, et al. Pflugers Arch. 2010 May;459(6):863-79. doi: 10.1007/s00424-010-0817-1. Epub 2010 Apr 11. Pflugers Arch. 2010. PMID: 20383718 Review. - Endothelium-Derived Hyperpolarization and Coronary Vasodilation: Diverse and Integrated Roles of Epoxyeicosatrienoic Acids, Hydrogen Peroxide, and Gap Junctions.
Ellinsworth DC, Sandow SL, Shukla N, Liu Y, Jeremy JY, Gutterman DD. Ellinsworth DC, et al. Microcirculation. 2016 Jan;23(1):15-32. doi: 10.1111/micc.12255. Microcirculation. 2016. PMID: 26541094 Free PMC article. Review.
Cited by
- Impact of High Salt Diet on Cerebral Vascular Function and Stroke in Tff3_-/-/_C57BL/6N Knockout and WT (C57BL/6N) Control Mice.
Kozina N, Mihaljević Z, Lončar MB, Mihalj M, Mišir M, Radmilović MD, Justić H, Gajović S, Šešelja K, Bazina I, Horvatić A, Matić A, Bijelić N, Rođak E, Jukić I, Drenjančević I. Kozina N, et al. Int J Mol Sci. 2019 Oct 19;20(20):5188. doi: 10.3390/ijms20205188. Int J Mol Sci. 2019. PMID: 31635131 Free PMC article. - Exercise training rescues impaired H2O2-mediated vasodilation in porcine collateral-dependent coronary arterioles through enhanced K+ channel activation.
Johnson KA, Jeffery E, Bray JF, Murphy MM, Heaps CL. Johnson KA, et al. Am J Physiol Heart Circ Physiol. 2023 May 1;324(5):H637-H653. doi: 10.1152/ajpheart.00710.2022. Epub 2023 Mar 3. Am J Physiol Heart Circ Physiol. 2023. PMID: 36867445 Free PMC article. - NOX5: Molecular biology and pathophysiology.
Touyz RM, Anagnostopoulou A, Rios F, Montezano AC, Camargo LL. Touyz RM, et al. Exp Physiol. 2019 May;104(5):605-616. doi: 10.1113/EP086204. Epub 2019 Mar 18. Exp Physiol. 2019. PMID: 30801870 Free PMC article. Review. - Calcium- and voltage-gated BK channels in vascular smooth muscle.
Dopico AM, Bukiya AN, Jaggar JH. Dopico AM, et al. Pflugers Arch. 2018 Sep;470(9):1271-1289. doi: 10.1007/s00424-018-2151-y. Epub 2018 May 11. Pflugers Arch. 2018. PMID: 29748711 Free PMC article. Review. - Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation.
Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Beyer AM, et al. Circ Res. 2016 Mar 4;118(5):856-66. doi: 10.1161/CIRCRESAHA.115.307918. Epub 2015 Dec 23. Circ Res. 2016. PMID: 26699654 Free PMC article.
References
- Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991;261:H1706–H1715. - PubMed
- Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circ. 1999;100:1951–7. - PubMed
- Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990;67:529–34. - PubMed
- Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+ -activated K+ channels. Circ. 2001;103:1992–8. - PubMed
- Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL080704-05/HL/NHLBI NIH HHS/United States
- R01 HL096647-01A2/HL/NHLBI NIH HHS/United States
- R01 HL096647-02/HL/NHLBI NIH HHS/United States
- R01 HL080704/HL/NHLBI NIH HHS/United States
- R01 HL094971/HL/NHLBI NIH HHS/United States
- R01 HL094971-04/HL/NHLBI NIH HHS/United States
- R01HL094971/HL/NHLBI NIH HHS/United States
- R01 HL096647/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous