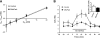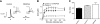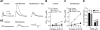Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease - PubMed (original) (raw)
Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease
Cameron H Good et al. FASEB J. 2011 Apr.
Abstract
Parkinson's disease (PD) involves progressive loss of nigrostriatal dopamine (DA) neurons over an extended period of time. Mitochondrial damage may lead to PD, and neurotoxins affecting mitochondria are widely used to produce degeneration of the nigrostriatal circuitry. Deletion of the mitochondrial transcription factor A gene (Tfam) in C57BL6 mouse DA neurons leads to a slowly progressing parkinsonian phenotype in which motor impairment is first observed at ~12 wk of age. L-DOPA treatment improves motor dysfunction in these "MitoPark" mice, but this declines when DA neuron loss is more complete. To investigate early neurobiological events potentially contributing to PD, we compared the neurochemical and electrophysiological properties of the nigrostriatal circuit in behaviorally asymptomatic 6- to 8-wk-old MitoPark mice and age-matched control littermates. Release, but not uptake of DA, was impaired in MitoPark mouse striatal brain slices, and nigral DA neurons lacked characteristic pacemaker activity compared with control mice. Also, hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channel function was reduced in MitoPark DA neurons, although HCN messenger RNA was unchanged. This study demonstrates altered nigrostriatal function that precedes behavioral parkinsonian symptoms in this genetic PD model. A full understanding of these presymptomatic cellular properties may lead to more effective early treatments of PD.
Figures
Figure 1.
SNc DA neurons from behaviorally asymptomatic MitoPark mice exhibit impaired HCN channel function. A) Neuron from a 7-wk-old MitoPark mouse filled with biocytin during whole-cell electrophysiological recording and counterstained for the DAT. B) I_h tail current analysis. Top left: voltage step protocol. Bottom left: series of currents recorded from a MitoPark DA neuron (red). Right: currents obtained from a SNc DA neuron from a control (DAT/DAT_cre Tfam/TfamloxP) 7-wk-old mouse (black) Scale bars = 100 ms; 500 pA. C) Frequency histogram showing _I_h amplitude at −140 mV for all DA neurons. Note the high number of SNc DA neurons from the MitoPark mice demonstrating little to no _I_h. D) Mean current-voltage relationship for _I_h in SNc DA neurons obtained from 7- to 8-wk-old control and MitoPark littermates. Also shown is the blockade of _I_h by ZD7288 (50 μM) in control DA neurons. E) Mean tail current conductance mediated by HCN channels on hyperpolarization (Gh). Note that Gh saturates at more depolarized membrane potentials and is much smaller in MitoPark DA neurons. F) Mean current-voltage relationship for normalized _I_h tail currents in SNc DA neurons from control and MitoPark mice. Note the significant rightward shift in the voltage dependence of _I_h in the MitoPark neurons. Type I, II, and III DA neurons were pooled for these analyses.
Figure 2.
HCN channel transcripts are unaltered in 6- to 10-wk-old MitoPark mice. Left panel: in situ hybridization of mRNA encoding HCN1–4 and TH in coronal sections were performed in control (c) and MitoPark (M) mice at 6, 10, and 21 wk of age. Note the decrease in TH mRNA at the level of the SNc/VTA at the 21 wk time point, although HCN levels were unaltered at any time point. Right panel: quantification of mRNA levels at 10 wk of age (_n_=4 pairs of control and MitoPark mice). TH, HCN2, and HCN3 mRNA levels were not significantly affected at this time point.
Figure 3.
SNc DA neurons from asymptomatic MitoPark mice show altered electrophysiological properties. Confirmed DAT+ neurons in the SNc of MitoPark mice were classified according to their firing frequencies. A) Mean current-voltage relationship for _I_h among type I (_n_=7), type II (_n_=13), and type 3 (_n_=12) cells. Note that type I cells were most similar to DA neurons (_n_=47) in control mice. B) Mean firing frequencies of SNc DA neurons. Note that both type II and type III neurons show both reduced _I_h and significant differences in baseline firing frequencies. No significant differences were observed between type I and cells from control mice (_P_>0.05, ANOVA). C) Mean resting membrane potentials of classified cell types. Type II cells were significantly more hyperpolarized and showed no spontaneous firing. D–G) Representative current clamp traces from control mice (D) and type I (E), type II (F), and type III (G) MitoPark mice. In F, a depolarizing current was applied during the time indicated by the dashed line in order to promote cell firing.
Figure 4.
Microdialysis experiments in 8-wk-old MitoPark mice reveal differences in DA release. A) No-net-flux experiments, plotting the net gain or loss of dialysate DA concentration (_C_in − _C_out) as a function of varying DA concentration in the perfusate C_in in control (DAT/DAT_cre Tfam/TfamloxP; open circles; _n_=6) and MitoPark (solid squares; _n_=5) mice. Neither the slope (extraction fraction _E_d), nor x intercept (basal extracellular DA) values differed significantly between the groups. B) Conventional microdialysis experiment comparing the effects of potassium (60 mM KCl) perfusion on DA release in the dorsal striatum of control and MitoPark mice. KCl-evoked overflow was significantly reduced in MitoPark mice, relative to controls (P<0.05; 2-way repeated-measures ANOVA). Inset: average basal DA levels during the 30-min period preceding KCl application. No differences were observed between groups (_P_>0.05; unpaired t test).
Figure 5.
Reduced dopamine release in behaviorally asymptomatic, 8-wk-old MitoPark mice. A) Representative voltammetric signals recorded in the dorsal striatum in brain slices obtained from an 8-wk-old control (DAT/DAT_cre Tfam/Tfam_loxp) and a MitoPark (DAT/DAT_cre Tfam_loxp/_Tfam_loxp) mouse. DA release was elicited by a single pulse, indicated by the arrowhead (300 μA, 1 ms). Insets: cyclic voltammogram obtained at the peak of the current is consistent with DA in each case. B) Summary of input-output curves for single-pulse release elicited in slices obtained from control and MitoPark mice (30 slices, 8 mice). Control 1 (18 slices, 5 mice) lacks the DATcre construct (DAT/DAT Tfam/Tfamloxp or DAT/DAT Tfam/Tfam), whereas control 2 mice contain the construct (11 slices, 3 mice; DAT/DAT_cre Tfam/Tfam_loxp). Signals were significantly reduced in the MitoPark mice relative to both control groups. ***P < 0.001; 2-way repeated-measures ANOVA. C) Summary of decay time constants (tau) for DA signals. A 1-way ANOVA revealed no significant differences (_P_=0.06) among the groups.
Figure 6.
Burst-induced DA release impairment in 8-wk-old MitoPark mice. A1) Representative voltammetric signals obtained in a control (DAT/DATcre Tfam/Tfamloxp) mouse by delivering either a single pulse (1p, solid line) or 10 pulses at 25 Hz (10p, shaded line). Traces represent an average of 3 signals obtained predrug (control), following nomifensine (5 μM, 10 min), and following TBZ (3 μM, 30 min). Note that the difference between signals is increased by the presence of nomifensine and that this is reversed by depletion of vesicles by TBZ. A2) Voltammetric signals from a MitoPark mouse before (control) and after nomifensine application. B) Summary of the relationship between the observed change in DA concentration per pulse in slices from control (DAT/DATcre Tfam/Tfamloxp, 14 slices, 4 mice), MitoPark (13 slices, 3 mice), and control slices treated with TBZ (11 slices, 4 mice). Slopes of the regression lines from MitoPark and control TBZ-treated slices were significantly reduced, relative to controls (P<0.05; 1-way ANOVA, Tukey's post hoc). _C_) In the presence of nomifensine, the slope of the regression line was significantly increased only in slices from control mice. Slopes from both MitoPark and control TBZ-treated slices (_n_=12 slices, 6 mice) did not differ significantly from each other (_P_>0.05; 1-way ANOVA, Tukey's post hoc). D) Concentration-dependent inhibition of DA signals by TBZ. Data are means ±
se
of the response 40 min following TBZ application at the indicated concentrations, normalized to the control (predrug) response. No significant differences were observed between slices from control and MitoPark mice at either concentration (number of slices given in parentheses; _P_>0.05, 2-way ANOVA).
Similar articles
- Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson's Disease.
Branch SY, Chen C, Sharma R, Lechleiter JD, Li S, Beckstead MJ. Branch SY, et al. J Neurosci. 2016 Apr 6;36(14):4026-37. doi: 10.1523/JNEUROSCI.1395-15.2016. J Neurosci. 2016. PMID: 27053209 Free PMC article. - Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity.
Langley MR, Ghaisas S, Ay M, Luo J, Palanisamy BN, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. Langley MR, et al. Neurotoxicology. 2018 Jan;64:240-255. doi: 10.1016/j.neuro.2017.06.002. Epub 2017 Jun 20. Neurotoxicology. 2018. PMID: 28595911 Free PMC article. - The MitoPark Mouse - an animal model of Parkinson's disease with impaired respiratory chain function in dopamine neurons.
Ekstrand MI, Galter D. Ekstrand MI, et al. Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S185-8. doi: 10.1016/S1353-8020(09)70811-9. Parkinsonism Relat Disord. 2009. PMID: 20082987 Review. - Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson's disease.
Langley MR, Ghaisas S, Palanisamy BN, Ay M, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. Langley MR, et al. Exp Neurol. 2021 Jul;341:113716. doi: 10.1016/j.expneurol.2021.113716. Epub 2021 Apr 8. Exp Neurol. 2021. PMID: 33839143 Free PMC article. - Voluntary exercise delays progressive deterioration of markers of metabolism and behavior in a mouse model of Parkinson's disease.
Lai JH, Chen KY, Wu JC, Olson L, Brené S, Huang CZ, Chen YH, Kang SJ, Ma KH, Hoffer BJ, Hsieh TH, Chiang YH. Lai JH, et al. Brain Res. 2019 Oct 1;1720:146301. doi: 10.1016/j.brainres.2019.146301. Epub 2019 Jun 18. Brain Res. 2019. PMID: 31226324 Free PMC article. Review.
Cited by
- Altered glutamate release in the dorsal striatum of the MitoPark mouse model of Parkinson's disease.
Farrand AQ, Gregory RA, Bäckman CM, Helke KL, Boger HA. Farrand AQ, et al. Brain Res. 2016 Nov 15;1651:88-94. doi: 10.1016/j.brainres.2016.09.025. Epub 2016 Sep 20. Brain Res. 2016. PMID: 27659966 Free PMC article. - Adenosine A(2A) Receptor Antagonists Do Not Disrupt Rodent Prepulse Inhibition: An Improved Side Effect Profile in the Treatment of Parkinson's Disease.
Bleickardt CJ, Lashomb AL, Merkel CE, Hodgson RA. Bleickardt CJ, et al. Parkinsons Dis. 2012;2012:591094. doi: 10.1155/2012/591094. Epub 2011 Dec 4. Parkinsons Dis. 2012. PMID: 22191072 Free PMC article. - Cypermethrin-induced nigrostriatal dopaminergic neurodegeneration alters the mitochondrial function: a proteomics study.
Agrawal S, Singh A, Tripathi P, Mishra M, Singh PK, Singh MP. Agrawal S, et al. Mol Neurobiol. 2015 Apr;51(2):448-65. doi: 10.1007/s12035-014-8696-7. Epub 2014 Apr 24. Mol Neurobiol. 2015. PMID: 24760363 - Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues.
Holroyd KB, Adrover MF, Fuino RL, Bock R, Kaplan AR, Gremel CM, Rubinstein M, Alvarez VA. Holroyd KB, et al. Neuropsychopharmacology. 2015 May;40(6):1495-509. doi: 10.1038/npp.2014.336. Epub 2014 Dec 30. Neuropsychopharmacology. 2015. PMID: 25547712 Free PMC article. - Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease.
Pickrell AM, Pinto M, Hida A, Moraes CT. Pickrell AM, et al. J Neurosci. 2011 Nov 30;31(48):17649-58. doi: 10.1523/JNEUROSCI.4871-11.2011. J Neurosci. 2011. PMID: 22131425 Free PMC article.
References
- Dauer W., Przedborski S. (2003) Parkinson's disease: mechanisms and models. Neuron 39, 889–909 - PubMed
- Mizuno Y., Sone N., Saitoh T. (1987) Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J. Neurochem. 48, 1787–1793 - PubMed
- Smeyne R. J., Jackson-Lewis V. (2005) The MPTP model of Parkinson's disease. Mol. Brain Res. 134, 57–66 - PubMed
- Bender A., Krishnan K. J., Morris C. M., Taylor G. A., Reeve A. K., Perry R. H., Jaros E., Hersheson J. S., Betts J., Klopstock T., Taylor R. W., Turnbull D. M. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 38, 515–517 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases