Clinical significance of microbial infection and adaptation in cystic fibrosis - PubMed (original) (raw)
Review
Clinical significance of microbial infection and adaptation in cystic fibrosis
Alan R Hauser et al. Clin Microbiol Rev. 2011 Jan.
Abstract
A select group of microorganisms inhabit the airways of individuals with cystic fibrosis. Once established within the pulmonary environment in these patients, many of these microbes adapt by altering aspects of their structure and physiology. Some of these microbes and adaptations are associated with more rapid deterioration in lung function and overall clinical status, whereas others appear to have little effect. Here we review current evidence supporting or refuting a role for the different microbes and their adaptations in contributing to poor clinical outcomes in cystic fibrosis.
Figures
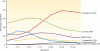
FIG. 1.
Prevalences of several common respiratory pathogens in CF as a function of age. (Adapted from the 2008 Annual Data Report of the Cystic Fibrosis Foundation Patient Registry, Bethesda, MD [
http://www.cff.org/UploadedFiles/research/ClinicalResearch/2008-Patient-Registry-Report.pdf
], with permission. © 2009 Cystic Fibrosis Foundation.)
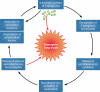
FIG. 2.
Cycle by which the presence of P. aeruginosa bacteria in the airways of individuals with CF leads to progressive pulmonary injury. In addition to directly damaging lung tissues, P. aeruginosa expresses factors that are recognized by the host immune system, resulting in release of proinflammatory cytokines. These cytokines cause the recruitment of large numbers of neutrophils that upon activation release elastase, collagenase, and oxygen radicals. The result is pulmonary injury as well as impaired bacterial clearance, which in turn leads to increased numbers of bacteria and exacerbation of the cycle.
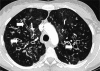
FIG. 3.
Chest computed tomography scan of an individual with CF showing bronchiectasis. Arrows indicate representative dilated airways that are characteristic of bronchiectasis. (Courtesy of Michelle Prickett.)
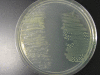
FIG. 4.
The mucoid phenotype of P. aeruginosa. The colonies on the left are a mucoid P. aeruginosa strain cultured from a CF patient. On the right, a nonmucoid variant of the same strain cultured from the same patient is shown.
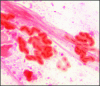
FIG. 5.
Gram-stained sputum specimen from a CF patient infected with mucoid P. aeruginosa. The orange material surrounding the bacteria is alginate (magnification, ×1,000). (Reprinted from reference with permission of the publisher. © 2007-2010 American Society for Clinical Pathology and © 2007-2010 American Journal of Clinical Pathology.)
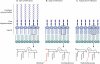
FIG. 6.
Modifications of P. aeruginosa LPS during CF respiratory infections. (A) Structures of LPSs from strains recovered from the environment, acute infections, or bronchiectasis. The lipid A, core oligosaccharide, and O-antigen polysaccharide components are indicated. In the lower panel, a more detailed structure of lipid A is shown. The large polygons represent the diglucosamine bisphosphate backbone of lipid A, and the staggered lines represent acyl groups. (The placement of the lipid A acyl chain shown in gray is unclear [177, 179].) (B) During early infection in individuals with CF, the O-antigen polysaccharide is frequently lost. Also, palmitate (shown as a red staggered line) and aminoarabinose (shown as a red hexagon) are added to the lipid A portion of LPS. (C) In patients with advanced CF, a hydroxydecanoate chain (shown as a red staggered line) is retained, likely due to mutations in the pagL gene, which encodes a lipid A deacylase (89, 175).
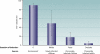
FIG. 7.
The proportion of P. aeruginosa isolates with functional type III secretion systems decreases with duration of infection in CF. TTS+, functional type III secretion system. Error bars indicate standard deviations. (Adapted from reference .)
FIG. 8.
Examples of P. aeruginosa colonies of normal morphology (left) and SCVs (right). (Reprinted from reference with permission of the publisher.)
Similar articles
- [Role of anaerobic flora in development of bronchopulmonary infection in children with mucoviscidosis].
Semykin SIu, Postnikov SS, Osipov GA. Semykin SIu, et al. Antibiot Khimioter. 2009;54(3-4):26-8. Antibiot Khimioter. 2009. PMID: 19711846 Russian. No abstract available. - Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease.
Döring G, Parameswaran IG, Murphy TF. Döring G, et al. FEMS Microbiol Rev. 2011 Jan;35(1):124-46. doi: 10.1111/j.1574-6976.2010.00237.x. FEMS Microbiol Rev. 2011. PMID: 20584083 Review. - Cystic fibrosis: A hereditary disease challenges microbiologists to a duel.
Heesemann J. Heesemann J. Int J Med Microbiol. 2010 Dec;300(8):513. doi: 10.1016/j.ijmm.2010.08.001. Int J Med Microbiol. 2010. PMID: 20940107 No abstract available. - Biogeochemical forces shape the composition and physiology of polymicrobial communities in the cystic fibrosis lung.
Quinn RA, Lim YW, Maughan H, Conrad D, Rohwer F, Whiteson KL. Quinn RA, et al. mBio. 2014 Mar 18;5(2):e00956-13. doi: 10.1128/mBio.00956-13. mBio. 2014. PMID: 24643867 Free PMC article. - Bacterial host interactions in cystic fibrosis.
Callaghan M, McClean S. Callaghan M, et al. Curr Opin Microbiol. 2012 Feb;15(1):71-7. doi: 10.1016/j.mib.2011.11.001. Epub 2011 Dec 1. Curr Opin Microbiol. 2012. PMID: 22137884 Review.
Cited by
- Phylogenetic analysis of burkholderia species by multilocus sequence analysis.
Estrada-de los Santos P, Vinuesa P, Martínez-Aguilar L, Hirsch AM, Caballero-Mellado J. Estrada-de los Santos P, et al. Curr Microbiol. 2013 Jul;67(1):51-60. doi: 10.1007/s00284-013-0330-9. Epub 2013 Feb 13. Curr Microbiol. 2013. PMID: 23404651 - The Variability of the Order Burkholderiales Representatives in the Healthcare Units.
Voronina OL, Kunda MS, Ryzhova NN, Aksenova EI, Semenov AN, Lasareva AV, Amelina EL, Chuchalin AG, Lunin VG, Gintsburg AL. Voronina OL, et al. Biomed Res Int. 2015;2015:680210. doi: 10.1155/2015/680210. Epub 2015 May 31. Biomed Res Int. 2015. PMID: 26114111 Free PMC article. - Rapid detection of four non-fermenting Gram-negative bacteria directly from cystic fibrosis patient's respiratory samples on the BD MAX™ system.
Rocchetti TT, Silbert S, Gostnell A, Kubasek C, Jerris R, Vong J, Widen R. Rocchetti TT, et al. Pract Lab Med. 2018 May 24;12:e00102. doi: 10.1016/j.plabm.2018.e00102. eCollection 2018 Nov. Pract Lab Med. 2018. PMID: 30009245 Free PMC article. - Paeonol Interferes With Quorum-Sensing in Pseudomonas aeruginosa and Modulates Inflammatory Responses In Vitro and In Vivo.
Tang H, Yang D, Zhu L, Shi F, Ye G, Guo H, Deng H, Zhao L, Xu Z, Li Y. Tang H, et al. Front Immunol. 2022 May 24;13:896874. doi: 10.3389/fimmu.2022.896874. eCollection 2022. Front Immunol. 2022. PMID: 35686124 Free PMC article.
References
- Aaron, S. D. 2006. Pseudomonas aeruginosa and cystic fibrosis—a nasty bug gets nastier. Respiration 73:16-17. - PubMed
- Aaron, S. D., K. Ramotar, W. Ferris, K. Vandemheen, R. Saginur, E. Tullis, D. Haase, D. Kottachchi, M. St. Denis, and F. Chan. 2004. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 169:811-815. - PubMed
- Abman, S. H., J. W. Ogle, N. Butler-Simon, C. M. Rumack, and F. J. Accurso. 1988. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J. Pediatr. 113:826-830. - PubMed
- Abman, S. H., J. W. Ogle, R. J. Harbeck, N. Butler-Simon, K. B. Hammond, and F. J. Accurso. 1991. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J. Pediatr. 119:211-217. - PubMed
- Adams, C., M. Morris-Quinn, F. McConnell, J. West, B. Lucey, C. Shortt, B. Cryan, J. B. G. Watson, and F. O'Gara. 1998. Epidemiology and clinical impact of P. aeruginosa infection in cystic fibrosis using AP-PCR fingerprinting. J. Infect. 37:151-158. - PubMed
Publication types
MeSH terms
Grants and funding
- K02 AI065615/AI/NIAID NIH HHS/United States
- R01 AI053674/AI/NIAID NIH HHS/United States
- R01 AI075191/AI/NIAID NIH HHS/United States
- R21 AI088286/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical