Microautophagy of cytosolic proteins by late endosomes - PubMed (original) (raw)
Microautophagy of cytosolic proteins by late endosomes
Ranjit Sahu et al. Dev Cell. 2011.
Erratum in
- Dev Cell. 2011 Mar 15;20(3):405-6
Abstract
Autophagy delivers cytosolic components to lysosomes for their degradation. The delivery of autophagic cargo to late endosomes for complete or partial degradation has also been described. In this report we present evidence that distinct autophagic mechanisms control cytosolic protein delivery to late endosomes and identify a microautophagy-like process that delivers soluble cytosolic proteins to the vesicles of late endosomes/multivesicular bodies (MVBs). This microautophagy-like process has selectivity and is distinct from chaperone-mediated autophagy that occurs in lysosomes. Endosomal microautophagy occurs during MVB formation, relying on the ESCRT I and III systems for formation of the vesicles in which the cytosolic cargo is internalized. Protein cargo selection is mediated by the chaperone hsc70 and requires the cationic domain of hsc70 for electrostatic interactions with the endosomal membrane. Therefore, we propose that endosomal microautophagy shares molecular components with both the endocytic and autophagic pathways.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
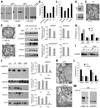
Figure 1. Cytosolic proteins are taken up by late endosomal compartments by mechanisms different from macroautophagy and CMA
(A) Western blot analysis for GAPDH, aldolase and cyclophilin in the indicated purified organelles. (B) Degradation (in percentage) of a radiolabeled pool of cytosolic proteins by the indicated intact organelles. Values are mean +S.E. (n = 4). (C) Effect of ATP depletion and GTP supplementation on the degradation of a radiolabeled pool of cytosolic proteins by late endosomes. Values were calculated as in B. (D) Western blot analysis for Atg7 of control (Ctr) and Atg7 knock down (Atg7-) DC. (E) Ultrastructural analysis of autophagosomes in Ctr DC. (F) Ultrastructural analysis of purified LE/MVB in Ctr and Atg7- DC. (G) Western blot analysis for cytosolic proteins and LAMP-2A of titrated amounts of purified LE from Ctr and Atg7- DC. Right: Densitometric analysis of blots as the one shown here (n= 3). (H) Degradation of a radiolabeled pool of cytosolic proteins by the indicated organelles isolated from Ctr and Atg7- DC. Values are mean +S.E. (n = 3). (I) Western blot analysis of GAPDH in LE or Exosomes (Ex) isolated from Ctr and Atg7- cells. (J) Western blot analysis for LAMP-2 isoforms and cytosolic proteins in fractions from control and LAMP-2A knock down DC. Right: Densitometric analysis of blots as the ones shown here (n = 3). (K) Ultrastructural analysis of LE/MVB compartments present in Ctr and LAMP-2A(−) cells. (L) Degradation of a radiolabeled pool of cytosolic proteins by LE and lysosomes (total, CMA+ and CMA−) from Ctr and LAMP-2A(−) DC. Values are mean +S.E. (n = 3). (M) Western blot analysis for the indicated proteins in LE isolated from Ctr (+) and LAMP-2A (−) NIH-3T3 fibroblasts. (*) Significant differences with control values. (See also Fig. S1 and 2).
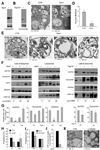
Figure 2. Incorporation of cytosolic proteins into late endosomes requires MVB formation
(A) Western blot analysis for Vps4 of control (+) and Vps4 knock down (−) DC. (B) Western blot analysis for Tsg101 of control (+) and Tsg101 knock down (−) DC. (C) Ultrastructural analysis of LE/MVB compartments present in Ctr and Vps4- cells. (D) Quantification of MVBs in Ctr and Vps4- cells. (*) Significant differences with control/none values. (E) Ultrastructural analysis of gradient purified LE/MVB from Ctr and Vps4- DC. (F) Western blot analysis for cytosolic proteins and LAMP-2A of titrated amount of gradient purified late endosomes and lysosomes from ctr, Vps4(−) and Tsg101(−)DC. (G) Densitometric analysis of western blots as the one show in f. (n = 3) (*) Significant differences with control/none values. (H) Degradation (in percentage) of a radiolabeled pool of cytosolic proteins by late endosomes and lysosomes isolated from Ctr and Vps4- cells. Values are mean +S.E. (n = 3). (I) Effect of preincubating endosomes or lysosomes with an antibody against Vps4 in their ability to transport/degrade a pool of radiolabeled cytosolic proteins measure as in h. Values are mean +S.E. (n = 3) (*) Significant differences with control/none values. (J) Endosomal uptake of cytosolic GFP. Data are expressed as percentage of endosomal uptake (measured by FACS on purified compartments) calculated over total cellular GFP. (*) Significant differences with control values. (K) Ultrastructural analysis of late endosomal multivesicular compartments present in control (CTR) and U18666A treated cells.
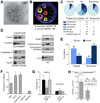
Figure 3. Specific cytosolic proteins are taken up by late endosomes in an hsc70-dependent manner
(A) Immunogold labeling for GAPDH present in a MVB isolated from DC. (B) Ultrastructural tomography of a LE/MVB depicting MA-mediated and microautophagy-mediated distribution of cytosolic GAPDH. (C) MS/MS analysis and percentage of protein distribution in the soluble cytosol fraction, in purified autophagic vacuoles (AV) and exosomes. (D) Western blot analysis of proteins detected in cytosol (Cyt), AV and exosomes (Exo). (E) Percentage of proteins containing a KFERQ-like motif distributed among cytosol and exosomes (F–G) Effect of preincubating intact LE with the indicated antibodies in their ability to degrade a pool of radiolabeled proteins (F) or radiolabeled GAPDH and cyclophilin (G). The inhibitory effect of the different antibodies on proteolysis rates, expressed as %, (F) or direct proteolysis values (G) are shown. Value are mean +S.E. (n = 3) (*) Significant differences with untreated fractions or control (#) Significant differences with GAPDH. (H) Sequences of wild type and amino acid substituted recombinant myc tagged GAPDH (n to a and r to a). Analysis of wild type and mutant GAPDHmyc levels in late endosomes. Data are reported as ratio between GAPDHmyc (wild type or mutated) in late endosomes (LE) versus total (Tot) in cells. (See also Fig. S3).
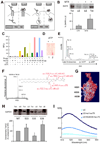
Figure 4. Recruitment of hsc70 to late endosomes is required for endosomal uptake of cytosolic proteins
(A) Western blot analysis of total (Tot) and endosomal (LE) DHFR upon cellular treatment without or with methotrexate (MTX). (B) Schematic of hsc70-mediated DHFR translocation in lysosomes (left) and late endosomes (right) in presence and absence of MTX. (C) Fluorescence emission scans (excitation wavelength 497 nm, emission scans 510–550 nm) of hsc70 binding to fluorescence labeled LE in presence or absence of ATP. Graphic representation of the fluorescence maximum emission (Λ 520 nm) extrapolated from data reported in Figure S4. Experimental conditions in c and S4 are shown using the same color code. (D) Thin layer chromatography (staining with 0.1% ninhydrin) of lipids eluted from hsc70/LE immunoprecipitation performed in presence or absence of ATP. (E) MS analysis of lipids eluted from hsc70/LE immunoprecipitation performed in presence or absence of ATP. (F) MS/MS fragmentation of (E) and sequenced phosphatidylserine fragments. A molecular species with an m/z of 786.36 (first peak of the molecular envelope) was detected both in total LE (positive control) and in the hsc70 immunoprecipitate but only in the absence of ATP. (G) Electrostatic surface of the C terminal of hsc70 using the color code built in the software Swiss PDB Viewer (red-negative charges (acidic), blue-positive charges (basic) and white-neutral (hydrophobic)). The NMR solved three-dimensional structure of the substrate-binding domain of the mammalian chaperone protein hsc70 was used for all modeling studies (PDB ID: Hsc70)(Morshauser et al., 1999). (H) Western blot analysis of hsc70-myc present in late endosomal compartments following transfection of wild type or mutated hsc70. Ratio between total and endosomal hsc70-myc is expressed. (I) Fluorescence analysis of wild type and mutant hsc70 binding to LE. (See also Fig. S4).
Comment in
- Shedding light on mammalian microautophagy.
Shpilka T, Elazar Z. Shpilka T, et al. Dev Cell. 2011 Jan 18;20(1):1-2. doi: 10.1016/j.devcel.2010.12.010. Dev Cell. 2011. PMID: 21238917 - Autophagy: ESCRTing proteins for microautophagy.
Wrighton KH. Wrighton KH. Nat Rev Mol Cell Biol. 2011 Mar;12(3):136-7. doi: 10.1038/nrm3075. Nat Rev Mol Cell Biol. 2011. PMID: 21346726 No abstract available.
Similar articles
- Differential activation of eMI by distinct forms of cellular stress.
Mesquita A, Glenn J, Jenny A. Mesquita A, et al. Autophagy. 2021 Aug;17(8):1828-1840. doi: 10.1080/15548627.2020.1783833. Epub 2020 Jul 9. Autophagy. 2021. PMID: 32559125 Free PMC article. - Selective endosomal microautophagy is starvation-inducible in Drosophila.
Mukherjee A, Patel B, Koga H, Cuervo AM, Jenny A. Mukherjee A, et al. Autophagy. 2016 Nov;12(11):1984-1999. doi: 10.1080/15548627.2016.1208887. Epub 2016 Aug 3. Autophagy. 2016. PMID: 27487474 Free PMC article. - Assessment of mammalian endosomal microautophagy.
Krause GJ, Cuervo AM. Krause GJ, et al. Methods Cell Biol. 2021;164:167-185. doi: 10.1016/bs.mcb.2020.10.009. Epub 2020 Nov 18. Methods Cell Biol. 2021. PMID: 34225914 Free PMC article. - Chaperone-mediated autophagy and endosomal microautophagy: Joint by a chaperone.
Tekirdag K, Cuervo AM. Tekirdag K, et al. J Biol Chem. 2018 Apr 13;293(15):5414-5424. doi: 10.1074/jbc.R117.818237. Epub 2017 Dec 15. J Biol Chem. 2018. PMID: 29247007 Free PMC article. Review. - ESCRT and autophagies: Endosomal functions and beyond.
Lefebvre C, Legouis R, Culetto E. Lefebvre C, et al. Semin Cell Dev Biol. 2018 Feb;74:21-28. doi: 10.1016/j.semcdb.2017.08.014. Epub 2017 Aug 12. Semin Cell Dev Biol. 2018. PMID: 28807884 Review.
Cited by
- Need an ESCRT for autophagosomal maturation?
Manil-Segalén M, Lefebvre C, Culetto E, Legouis R. Manil-Segalén M, et al. Commun Integr Biol. 2012 Nov 1;5(6):566-71. doi: 10.4161/cib.21522. Commun Integr Biol. 2012. PMID: 23336026 Free PMC article. - SUMO-1 is associated with a subset of lysosomes in glial protein aggregate diseases.
Wong MB, Goodwin J, Norazit A, Meedeniya AC, Richter-Landsberg C, Gai WP, Pountney DL. Wong MB, et al. Neurotox Res. 2013 Jan;23(1):1-21. doi: 10.1007/s12640-012-9358-z. Epub 2012 Nov 15. Neurotox Res. 2013. PMID: 23229893 - Tissue-specific knockout in the Drosophila neuromuscular system reveals ESCRT's role in formation of synapse-derived extracellular vesicles.
Chen X, Perry S, Fan Z, Wang B, Loxterkamp E, Wang S, Hu J, Dickman D, Han C. Chen X, et al. PLoS Genet. 2024 Oct 10;20(10):e1011438. doi: 10.1371/journal.pgen.1011438. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39388480 Free PMC article. - Autophagy and organelle homeostasis in cancer.
Miller DR, Thorburn A. Miller DR, et al. Dev Cell. 2021 Apr 5;56(7):906-918. doi: 10.1016/j.devcel.2021.02.010. Epub 2021 Mar 8. Dev Cell. 2021. PMID: 33689692 Free PMC article. Review. - The Lysosome as a Regulatory Hub.
Perera RM, Zoncu R. Perera RM, et al. Annu Rev Cell Dev Biol. 2016 Oct 6;32:223-253. doi: 10.1146/annurev-cellbio-111315-125125. Epub 2016 Aug 3. Annu Rev Cell Dev Biol. 2016. PMID: 27501449 Free PMC article. Review.
References
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3- phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–10470. - PubMed
- Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem. 2000;275:30839–30843. - PubMed
- Castellino F, Germain RN. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73–88. - PubMed
- Chiang H, Terlecky S, Plant C, Dice J. A role for a 70 kDa heat shock protein in lysosomal degradation of intracellular protein. Science. 1989;246:382–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK041918-20/DK/NIDDK NIH HHS/United States
- DK041918/DK/NIDDK NIH HHS/United States
- P01 DK041918/DK/NIDDK NIH HHS/United States
- R01 AI048832-06/AI/NIAID NIH HHS/United States
- R01 AI048833/AI/NIAID NIH HHS/United States
- R01 AG045223/AG/NIA NIH HHS/United States
- S10 RR019352/RR/NCRR NIH HHS/United States
- R01 AI048832/AI/NIAID NIH HHS/United States
- P01 AG031782-03/AG/NIA NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- T32 AG023475/AG/NIA NIH HHS/United States
- AG031782/AG/NIA NIH HHS/United States
- T32 AG023475-08/AG/NIA NIH HHS/United States
- AI48833/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous