Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses - PubMed (original) (raw)
Review
Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses
Glen N Barber. Curr Opin Immunol. 2011 Feb.
Abstract
The early detection of microbes is the responsibility of the innate immune system which has evolved to sense pathogen derived molecules such as lipopolysaccharides and non-self nucleic acid, to trigger host defense countermeasures. These sensors include the RIG-I-like helicase (RLH) family that specifically recognizes viral RNA, as well as the cytoplasmic, nucleotide binding oligermerization domain (NOD)-like receptor and Toll-like receptor (TLR) pathways that sense a variety of microbial derived molecules. Comprehending how the cell senses foreign DNA, generated by certain viruses, bacteria and possibly parasites has proven elusive but is of significant importance since such information could shed insight into the causes of microbial related disease, including viral associated cancers and autoimmune disorders. Plasmacytoid dendritic cells are known to utilize TLR9 to detect pathogen-associated DNA and to trigger the production of type I interferon (IFN), as well as other cytokines, although alternate key DNA detecting sensors remain to be identified. Recently however, a molecule referred to as AIM2 (absent in melanoma 2) was found to be essential for mediating inflammatory reactions triggered by cytoplasmic DNA. In addition, an endoplasmic reticulum associated protein referred to as STING (for stimulator of interferon genes) was demonstrated as being pivotal for facilitating IFN production in response to intracellular DNA and a variety of DNA pathogens. Here, we review recent discoveries relating to the detection of foreign DNA, including the importance of the STING and AIM2 and the activation of innate signaling pathways.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
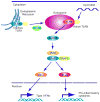
Fig 1. TLR9-dependent innate immune signaling
Latent TLR9 resides in the ER and traffics to endosomes where it is processed to become active. Trafficking is UNC-93B-dependent. Cytoplasmic CpG DNA engulfed in endolysosomes associates with TLR9 which triggers recruitment of a signaling complex consisting of MyD88, IRAK4 and TRAF6. TRAF6 in turn activates TAK1, and subsequently MAPK and the IKK complex (IKKα, IKKβ, and IKKγ) to activate NF-κB. NF-κB and MAPK regulates inflammatory cytokine expression. IRAK4 also activates TRAF3 and IRAK1, which catalyzes IRF7 phosphorylation to induce type I IFNs expression.
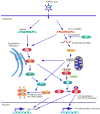
Fig 2. Cytoplasmic DNA-mediated IFN production
Cytosolic AT-rich dsDNA is recognized by RNA PolIII and can be transcribed into 5′triphosphate RNA which activates RIG-I/IPS-1 signaling. IPS1 interacts with FADD/RIP1 which activate NF-κB. IPS-1 also activates TBK1 which phosphorylates IRF-3 and induces type I IFN expression. AT-rich dsDNA or non-AT-rich dsDNA is also recognized by unknown receptor(s) and activates STING-dependent signaling. STING localizes in the ER with the translocon complex and activates TBK1 to induce type I IFN expression. STING also activates NF-κB to induce pro-inflammatory cytokine responses.
Fig 3. STING is associated with the translocon
STING associates with the translocon associated protein (TRAP) complex comprising four subunits (TRAP α–Δ) responsible for facilitating translocation of proteins from ER associated ribosomes, into the ER lumen following translation. The TRAP complex is known to associate with three subunits, SEC61α, SEC61β and SEC61γ. TRAP associated STING is essential for DNA-mediated cytoplasmic IFN production triggered by DNA sensor(s). STING also facilitates RIG-I-mediated signaling perhaps through IPS-1 associated with mitochondria bound to the ER (MAM). STING function was found to be inhibited by certain flavivirus proteins.
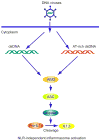
Figure 4
AIM2-dependent signaling and the inflammatory responses: Cytosolic DNA binds to the HIN-200 domains of AIM2 and activates the ASC/Caspase1 inflammasome pathway to produce IL-1β.
Similar articles
- Cytoplasmic DNA innate immune pathways.
Barber GN. Barber GN. Immunol Rev. 2011 Sep;243(1):99-108. doi: 10.1111/j.1600-065X.2011.01051.x. Immunol Rev. 2011. PMID: 21884170 Review. - The STING pathway and regulation of innate immune signaling in response to DNA pathogens.
Ishikawa H, Barber GN. Ishikawa H, et al. Cell Mol Life Sci. 2011 Apr;68(7):1157-65. doi: 10.1007/s00018-010-0605-2. Epub 2010 Dec 15. Cell Mol Life Sci. 2011. PMID: 21161320 Free PMC article. Review. - Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review. - The STING controlled cytosolic-DNA activated innate immune pathway and microbial disease.
Konno H, Barber GN. Konno H, et al. Microbes Infect. 2014 Dec;16(12):998-1001. doi: 10.1016/j.micinf.2014.10.002. Epub 2014 Oct 18. Microbes Infect. 2014. PMID: 25449752 Free PMC article. Review. - STING signaling and host defense against microbial infection.
Ahn J, Barber GN. Ahn J, et al. Exp Mol Med. 2019 Dec 11;51(12):1-10. doi: 10.1038/s12276-019-0333-0. Exp Mol Med. 2019. PMID: 31827069 Free PMC article. Review.
Cited by
- The type I interferon system in the etiopathogenesis of autoimmune diseases.
Rönnblom L. Rönnblom L. Ups J Med Sci. 2011 Nov;116(4):227-37. doi: 10.3109/03009734.2011.624649. Ups J Med Sci. 2011. PMID: 22066971 Free PMC article. Review. - The C-Terminal Tail of TRIM56 Dictates Antiviral Restriction of Influenza A and B Viruses by Impeding Viral RNA Synthesis.
Liu B, Li NL, Shen Y, Bao X, Fabrizio T, Elbahesh H, Webby RJ, Li K. Liu B, et al. J Virol. 2016 Apr 14;90(9):4369-4382. doi: 10.1128/JVI.03172-15. Print 2016 May. J Virol. 2016. PMID: 26889027 Free PMC article. - A STING-dependent innate-sensing pathway mediates resistance to corneal HSV-1 infection via upregulation of the antiviral effector tetherin.
Royer DJ, Carr DJ. Royer DJ, et al. Mucosal Immunol. 2016 Jul;9(4):1065-75. doi: 10.1038/mi.2015.124. Epub 2015 Dec 2. Mucosal Immunol. 2016. PMID: 26627457 Free PMC article. - Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) in fish: current knowledge and future perspectives.
Chen SN, Zou PF, Nie P. Chen SN, et al. Immunology. 2017 May;151(1):16-25. doi: 10.1111/imm.12714. Epub 2017 Feb 28. Immunology. 2017. PMID: 28109007 Free PMC article. Review. - STING Negatively Regulates Double-Stranded DNA-Activated JAK1-STAT1 Signaling via SHP-1/2 in B Cells.
Dong G, You M, Ding L, Fan H, Liu F, Ren D, Hou Y. Dong G, et al. Mol Cells. 2015 May;38(5):441-51. doi: 10.14348/molcells.2015.2359. Epub 2015 May 7. Mol Cells. 2015. PMID: 25947293 Free PMC article.
References
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. - PubMed
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials