Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis - PubMed (original) (raw)
Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis
Benjamin D Solomon et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Neuropilin-1 (Nrp1) is a cell surface molecule originally identified for its role in neuronal development. Recently, Nrp1 has been implicated in several aspects of immune function including maintenance of the immune synapse and development of regulatory T (T(reg)) cells. In this study, we provide evidence for a central role of Nrp1 in the regulation of CD4 T-cell immune responses in experimental autoimmune encephalitis (EAE). EAE serves as an animal model for the central nervous system (CNS) inflammatory disorder multiple sclerosis (MS). EAE is mediated primarily by CD4(+) T cells that migrate to the CNS and mount an inflammatory attack against myelin components, resulting in CNS pathology. Using a tissue-specific deletion system, we observed that the lack of Nrp1 on CD4(+) T cells results in increased EAE severity. These conditional knockout mice exhibit preferential T(H)-17 lineage commitment and decreased T(reg)-cell functionality. Conversely, CD4(+) T cells expressing Nrp1 suppress effector T-cell proliferation and cytokine production both in vivo and in vitro independent of T(reg) cells. Nrp1-mediated suppression can be inhibited by TGF-β blockade but not by IL-10 blockade. These results suggest that Nrp1 is essential for proper maintenance of peripheral tolerance and its absence can result in unchecked autoreactive responses, leading to diseases like EAE and potentially MS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
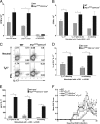
Nrp1 expression is protective against EAE, whereas the lack of Nrp1 increases disease severity. (A) WT mice were ECi with 100 μg of MOG35–55 (n = 6) or PBS (n = 4) and immunized with MOG35–55/CFA plus pertussis toxin to induce EAE. Representative (1 of 6) results are expressed as mean EAE score (±SEM, *P < 0.05). (B) CD4+CD25− T cells were isolated from Ac1-11–activated MBP-TCR-Tg mice, transduced with a retroviral GFP construct containing Nrp1 (circle, n = 3) or an empty vector (square, n = 3), and 106 cells were adoptively transferred into B10.Pl _TCR_α−/− recipient mice concomitant with 106 (untransduced) Ac1-11–activated CD4+CD25− cells. Untransduced cells served as a control (triangle, n = 3). Results from one experiment are expressed as mean EAE score (±SEM, #P < 0.05 for Nrp1+GFP+ transduced vs. GFP+ transduced controls; *P < 0.05 for both Nrp1+GFP+ transduced vs. GFP+ transduced and Nrp1+GFP+ transduced vs. untransduced controls). (C) EAE was induced by using MOG35–55/CFA plus pertussis toxin in Nrp1flx/flxCD4Cre+ (n = 5) and WT mice (n = 5). Representative (1 of 4) results are expressed as mean EAE score (±SEM, *P < 0.05). (D) CD4+ cells from Nrp1flx/flxCD4Cre+ (n = 20) and WT mice (n = 5) primed s.c. with a MOG35–55/CFA emulsion were isolated and transferred into C57BL/6-_TCR_α−/− recipient mice (n = 4 mice each) followed by immunization using MOG35–55/CFA plus pertussis toxin to induce EAE. Results from one experiment are displayed as mean (±SEM) EAE score.
Fig. 2.
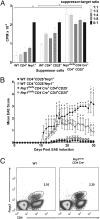
_Nrp1_-deficient CD4+ T cells display an increased TH-17 phenotype and are more proliferative than WT. (A) Naïve CD4+ cells [WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars), n = 5 each] were skewed toward TH-17. After 7 d, skewed cells were stimulated with either APC and MOG, or with anti-CD3 and anti-CD28, or were left unskewed and unstimulated. Representative (1 of 5) results are displayed as mean (±SEM) CPM × 103 (*P < 0.05). (B) EAE was induced by using MOG35–55/CFA plus pertussis toxin in WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars) mice (n ≥ 3 each). On day 30, CD4+ cells were stimulated with 10 μg/mL MOG and cultured under neutral, TH-17 polarizing, or TH-17 polarizing with supplemental IL-23 conditions. Representative results (1 of 3) are displayed as mean (±SEM) cpm × 103. (*P < 0.05). (C) Naïve CD4+ T cells (WT and Nrp1flx/flxCD4Cre+, n = 4 mice each), were cultured under neutral or TH-17 polarizing conditions. Cells were stained for CD4, IFNγ, and IL-17 and analyzed by flow cytometry. Data are representative of three experiments. (D) Naïve CD4+ cells [WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars)] were cultured under neutral or TH-17 polarizing conditions, stained for CD4 and IL-17 expression, and analyzed by flow cytometry. Representative (1 of 5) results are presented as mean (±SEM) percent of total CD4+ cells, which are IL-17+ (*P < 0.05). (E) Naïve CD4+ cells [WT (gray bars) and Nrp1flx/flxCD4Cre+ (black bars), n = 5 each] were cultured under neutral or TH-17 polarizing conditions, and cell culture supernatant was analyzed by ELISA for IL-17. Representative (1 of 5) results are displayed as mean (±SEM) IL-17 pg/mL (*P ≤ 0.005). (F) WT (squares) or Nrp1flx/flxCD4Cre+ (circles) mice (n = 5 each) immunized for EAE by using MOG35–55/CFA plus pertussis toxin were treated with (open symbols) or without (filled symbols) anti–TH-17 antibodies (anti–IL-6, anti–IL-23, anti–TGF-β). Representative (1 of 2) results are presented as mean EAE score ± SEM (#P < 0.05 for the Nrp1flx/flxCD4Cre+ versus the Nrp1flx/flxCD4Cre+-anti–TH-17 treated group. *P < 0.05 for the WT versus WT anti–TH-17 group.)
Fig. 3.
Nrp1 deficiency impairs Treg cell function. (A) The 2D2-Tg (17) CD4+ cells (n ≥ 3 mice) were used as target cells. Naïve Nrp1flx/flxCD4Cre+ or WT CD4+CD25+ cells (n ≥ 7 each), as well as WT CD4+CD25−Nrp1+ cells (all purified by using magnetic beads), were used as suppressor T cells. Cells were stimulated with 10 μg/mL MOG and APC (5:1 APC:target ratio), cultured for 48 h, then pulsed with 0.5 μCi/well Td-3H for 18 h. P values compare either WT CD4+Nrp1+ (*P < 0.05) or WT CD4+CD25+ (+P < 0.05) suppressor cells to Nrp1flx/flxCD4Cre+ CD4+CD25+ cells of the same ratio. Representative (1 of 4) results are expressed in mean (±SEM) CPM × 103. (B) The 2D2-Tg CD4+ T cells (n = 5 mice) were skewed to TH-17 in vitro, and 107 cells were transferred into C57BL/6-TCR-α−/− recipients along with: 106 naïve WT Nrp1+CD4+CD25+, or Nrp1+CD4+CD25−; or, naïve Nrp1flx/flxCD4Cre+ CD4+CD25+ or CD4+CD25− cells (n = 10 mice each). EAE was then induced in recipients by using MOG35–55/CFA plus pertussis toxin. Results from one experiment are expressed as mean (± SEM) EAE score. P values compare: WT CD4+CD25−Nrp1+ versus either _Nrp1flx/flxCD4Cre+_CD4+CD25+ or _Nrp1flx/flxCD4Cre+_CD4+CD25−, #P < 0.05; WT CD4+CD25+Nrp1+ versus either _Nrp1flx/flxCD4Cre+_CD4+CD25+ or _Nrp1flx/flxCD4Cre+_CD4+CD25−, *P < 0.05; _Nrp1flx/flxCD4Cre+_CD4+CD25+ versus _Nrp1flx/flxCD4Cre+_CD4+CD25−, +P < 0.05). (C) CD4+ T cells from WT and Nrp1flx/flxCD4Cre+ mice (n ≥ 3) were isolated, stained for CD4 and Foxp3, and analyzed by FACS. Results are representative of three experiments.
Fig. 4.
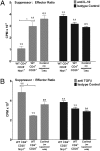
Suppression by CD4+Nrp1+ cells is abrogated in the presence of anti–TGF-β but not anti–IL-10. (A) The 2D2-Tg CD4+ T cells (n ≥ 3 mice) were primed in vivo, isolated by using magnetic beads, and used as target cells. Naïve WT and Nrp1flx/flxCD4Cre+ CD4+CD25+ T cells (n = 10) and naïve WT CD4+CD25−Nrp1+ T cells (n = 10) were isolated by using magnetic beads and used as suppressor T cells. Suppressors and targets were combined at a 1:1 ratio. Cells were treated with anti–IL-10 (10 μg/mL) or an isotype control (10 μg/mL) and stimulated with 10 μg/mL MOG and APC (5:1 APC:target ratio). Representative (1 of 3) results are expressed as mean (±SEM) CPM × 103. (WT CD4+CD25+ versus WT CD4+CD25−Nrp1+, *P < 0.05.) (B) Cell populations were purified, combined, and cultured as in Fig. 4_A_, except cells were treated with anti–TGF-β (10 μg/mL) or an isotype control (10 μg/mL). Representative (1 of 3) results are expressed as mean (±SEM) cpm × 103. (WT CD4+CD25+ versus WT CD4+CD25−Nrp1+, *P < 0.05.)
Similar articles
- Opioid growth factor and low-dose naltrexone impair central nervous system infiltration by CD4 + T lymphocytes in established experimental autoimmune encephalomyelitis, a model of multiple sclerosis.
Hammer LA, Waldner H, Zagon IS, McLaughlin PJ. Hammer LA, et al. Exp Biol Med (Maywood). 2016 Jan;241(1):71-8. doi: 10.1177/1535370215596384. Epub 2015 Jul 22. Exp Biol Med (Maywood). 2016. PMID: 26202376 Free PMC article. - Chronological changes of CD4(+) and CD8(+) T cell subsets in the experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis.
Sonobe Y, Jin S, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Sonobe Y, et al. Tohoku J Exp Med. 2007 Dec;213(4):329-39. doi: 10.1620/tjem.213.329. Tohoku J Exp Med. 2007. PMID: 18075237 - Lack of junctional adhesion molecule (JAM)-B ameliorates experimental autoimmune encephalomyelitis.
Tietz S, Périnat T, Greene G, Enzmann G, Deutsch U, Adams R, Imhof B, Aurrand-Lions M, Engelhardt B. Tietz S, et al. Brain Behav Immun. 2018 Oct;73:3-20. doi: 10.1016/j.bbi.2018.06.014. Epub 2018 Jun 18. Brain Behav Immun. 2018. PMID: 29920328 - Emerging concepts in autoimmune encephalomyelitis beyond the CD4/T(H)1 paradigm.
Batoulis H, Addicks K, Kuerten S. Batoulis H, et al. Ann Anat. 2010 Aug 20;192(4):179-93. doi: 10.1016/j.aanat.2010.06.006. Epub 2010 Jul 15. Ann Anat. 2010. PMID: 20692821 Review. - Regulatory T cells in spontaneous autoimmune encephalomyelitis.
Furtado GC, Olivares-Villagómez D, Curotto de Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Furtado GC, et al. Immunol Rev. 2001 Aug;182:122-34. doi: 10.1034/j.1600-065x.2001.1820110.x. Immunol Rev. 2001. PMID: 11722629 Review.
Cited by
- Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth.
Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, Schadendorf D, Buer J, Helfrich I. Hansen W, et al. J Exp Med. 2012 Oct 22;209(11):2001-16. doi: 10.1084/jem.20111497. Epub 2012 Oct 8. J Exp Med. 2012. PMID: 23045606 Free PMC article. - Neuropilin-1 Expression on CD4 T Cells Is Atherogenic and Facilitates T Cell Migration to the Aorta in Atherosclerosis.
Gaddis DE, Padgett LE, Wu R, Hedrick CC. Gaddis DE, et al. J Immunol. 2019 Dec 15;203(12):3237-3246. doi: 10.4049/jimmunol.1900245. Epub 2019 Nov 18. J Immunol. 2019. PMID: 31740486 Free PMC article. - Genetic status of KRAS influences Transforming Growth Factor-beta (TGF-β) signaling: An insight into Neuropilin-1 (NRP1) mediated tumorigenesis.
Vivekanandhan S, Mukhopadhyay D. Vivekanandhan S, et al. Semin Cancer Biol. 2019 Feb;54:72-79. doi: 10.1016/j.semcancer.2018.01.014. Epub 2018 Feb 2. Semin Cancer Biol. 2019. PMID: 29409705 Free PMC article. Review. - Regulatory T Cells in Allergy and Asthma.
Martín-Orozco E, Norte-Muñoz M, Martínez-García J. Martín-Orozco E, et al. Front Pediatr. 2017 May 23;5:117. doi: 10.3389/fped.2017.00117. eCollection 2017. Front Pediatr. 2017. PMID: 28589115 Free PMC article. Review. - The crosstalk between neuropilin-1 and tumor necrosis factor-α in endothelial cells.
Wang Y, Wang E, Anany M, Füllsack S, Huo YH, Dutta S, Ji B, Hoeppner LH, Kilari S, Misra S, Caulfield T, Vander Kooi CW, Wajant H, Mukhopadhyay D. Wang Y, et al. Front Cell Dev Biol. 2024 Jun 27;12:1210944. doi: 10.3389/fcell.2024.1210944. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38994453 Free PMC article.
References
- Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med. 2002;53:285–302. - PubMed
- Niino M, Fukazawa T, Kikuchi S, Sasaki H. Recent advances in genetic analysis of multiple sclerosis: Genetic associations and therapeutic implications. Expert Rev Neurother. 2007;7:1175–1188. - PubMed
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–513. - PubMed
- Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22 AI057854/AI/NIAID NIH HHS/United States
- R56 AI072434/AI/NIAID NIH HHS/United States
- AI 072434-01A2/AI/NIAID NIH HHS/United States
- AI 57854/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous