HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration - PubMed (original) (raw)
HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration
Hui-Ming Gao et al. J Neurosci. 2011.
Abstract
What drives the gradual degeneration of dopamine neurons in Parkinson's disease (PD), the second most common neurodegenerative disease, remains elusive. Here, we demonstrated, for the first time, that persistent neuroinflammation was indispensible for such a neurodegenerative process. 1-Methyl-4-phenylpyridinium, lipopolysaccharide (LPS), and rotenone, three toxins often used to create PD models, produced acute but nonprogressive neurotoxicity in neuron-enriched cultures. In the presence of microglia (brain immune cells), these toxins induced progressive dopaminergic neurodegeneration. More importantly, such neurodegeneration was prevented by removing activated microglia. Collectively, chronic neuroinflammation may be a driving force of progressive dopaminergic neurodegeneration. Conversely, ongoing neurodegeneration sustained microglial activation. Microglial activation persisted only in the presence of neuronal damage in LPS-treated neuron-glia cultures but not in LPS-treated mixed-glia cultures. Thus, activated microglia and damaged neurons formed a vicious cycle mediating chronic, progressive neurodegeneration. Mechanistic studies indicated that HMGB1 (high-mobility group box 1), released from inflamed microglia and/or degenerating neurons, bound to microglial Mac1 (macrophage antigen complex 1) and activated nuclear factor-κB pathway and NADPH oxidase to stimulate production of multiple inflammatory and neurotoxic factors. The treatment of microglia with HMGB1 led to membrane translocation of p47(phox) (a cytosolic subunit of NADPH oxidase) and consequent superoxide release, which required the presence of Mac1. Neutralization of HMGB1 and genetic ablation of Mac1 and gp91(phox) (the catalytic submit of NADPH oxidase) blocked the progressive neurodegeneration. Our findings indicated that HMGB1-Mac1-NADPH oxidase signaling axis bridged chronic neuroinflammation and progressive dopaminergic neurodegeneration, thus identifying a mechanistic basis for chronic PD progression.
Figures
Figure 1.
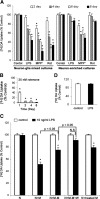
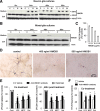
Progressive DA neurodegeneration depended on the presence of microglia. A, Time course dependence for the effect of LPS, MPP+, and rotenone on DA uptake capacity in the mesencephalic neuron–glia cultures but not in the neuron-enriched cultures. Cultures were treated with vehicle, 10 ng/ml LPS, 0.5 μ
m
MPP+, or 10 n
m
rotenone for 2–8 d and then assayed for [3H]DA uptake. After 2, 4, 6, and 8 d posttreatment, the amount of DA uptake in vehicle-treated neuron–glia cultures was 0.79 ± 0.14, 0.81 ± 0.04, 0.690 ± 0.10, and 0.60 ± 0.06 pmol/min per well, respectively; in vehicle-treated neuron-enriched cultures, it was 0.88 ± 0.06, 0.86 ± 0.15, 0.82 ± 0.10, and 0.79 ± 0.11 pmol/min per well, respectively. B, Treatment of neuron-enriched cultures with a high dose of rotenone (25 n
m
) caused a quick and dramatic, but nonprogressive, reduction in DA uptake. C, D, Removal of activated microglia rescued DA neurons from progressive degeneration. The neuron-enriched cultures (N) and reconstituted cultures (N + M) containing microglia (5 × 104 microglia/well) in transwells and enriched neuron layer underneath were treated with vehicle or 10 ng/ml LPS for 24 h. The transwells with treated microglia were then removed from the original culture plates (N + M − M) and put into sister culture plates with existing neuron-enriched cultures (N + treated M). The survival of DA neurons was assessed by [3H]DA uptake immediately (D) or 6 d later (C). The results are expressed as a percentage of the time-matched control cultures and are the mean ± SEM of three to four experiments performed in triplicate. *p < 0.05 compared with the time-matched vehicle-treated controls (A, B) or the corresponding vehicle-treated controls (C, D). p < 0.05 was considered statistically significant. Rot, Rotenone; M, microglia; N + M − M + W, wash the neuron layer after the removal of transwells; N.S., not significant.
Figure 2.
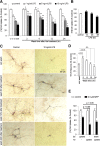
Effects of washout of LPS/MPP+ and released inflammatory/neurotoxic factors on DA neurodegeneration. A, D, At specified time points, the culture media were completely aspirated and the neuron–glia cultures were washed twice and replenished with fresh treatment media. DA neuron survival was determined with [3H]DA uptake assay (A) or quantification of TH-IR neurons (D) 7 d after the initial treatment. Compared with time-matched controls, LPS-treated cultures showed significant neuronal damage even after the wash. B, There was no significant reduction in [3H]DA uptake within 48 h after LPS treatment of neuron–glia cultures with 10 ng/ml LPS. C, Representative images from three experiments confirmed the neuronal loss and dendrite degeneration of DA neurons even after drug washout and inflammatory factor withdrawal at different time points. E, Neuron–glia cultures were treated with vehicle or 0.5 μ
m
MPP+. Two days after the treatment, the cells were washed twice and refed with fresh treatment media. DA uptake assay was performed 2 or 7 d after the initial treatment. Notably, the drug washout at 2 d after MPP+ treatment could not stop DA neurons from additional degeneration. Results are expressed as a percentage of the time-matched controls and are the mean ± SEM of four experiments performed in triplicate. *p < 0.05 compared with the time-matched vehicle-treated controls. #p < 0.05 compared with the LPS-treated cultures without wash. p < 0.05 was considered statistically significant. N.S., Not significant.
Figure 3.
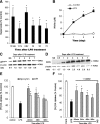
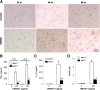
Long-lasting secretion of superoxide and NO from activated microglia in mediating progressive DA neurodegeneration. A, B, Continuing release of superoxide and nitrite (an indicator of NO production) in midbrain neuron–glia cultures after LPS treatment. The release of superoxide was measured as SOD-inhibitable WST-1 reduction. *p < 0.05 compared with the time-matched control cultures. C, D, Persistent upregulation of gp91phox and iNOS was observed after the neuron–glia cultures were treated with LPS. The ratios of densitometry values of gp91phox and iNOS to those of β-actin were analyzed and averaged from three experiments. E, F, NADPH oxidase inhibitors apocynin or DPI and iNOS inhibitor 1400W protected DA neurons against LPS-mediated chronic neurodegeneration, when neuron–glia cultures were pretreated for 30 min, or posttreated at 48 h. Here, DA uptake was conducted 7 d after initial LPS treatment. *p < 0.05 compared with the corresponding LPS-treated cultures. Results shown above are the mean ± SEM of three to four experiments performed in triplicate. NS, Normal saline.
Figure 4.
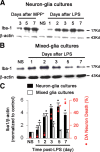
Ongoing neurodegeneration sustained microglial activation. A–C, The neuron–glia cultures (A, C) and mixed-glia cultures (B, C) were treated with normal saline (NS), 0.5 μ
m
MPP+, or 10 ng/ml LPS for different periods of time and were lysed in RIPA buffer. Cell lysates were size fractionated by 4–12% SDS-PAGE gels and probed by Western blot for Iba1 or β-actin. C, The ratios of densitometry values of Iba1 and β-actin in A and B were analyzed and normalized to each responsive control. Quantification of TH-IR neurons in LPS-treated neuron–glia cultures revealed time-dependent loss of DA neurons. The long-term upregulation of Iba-1 positively correlated with the neuronal death. The results are the mean ± SEM of three experiments performed in triplicate. *p < 0.05 compared with the corresponding saline-treated controls.
Figure 5.
HMGB1 mediated persistent crosstalk between activated microglia and degenerating neurons. A, B, The neuron–glia cultures (A) and mixed-glia cultures (B) were treated with normal saline (NS), 10 ng/ml LPS, 10 n
m
rotenone, or 0.5 μ
m
MPP+. The culture media were collected at indicated time points and concentrated with Amicon Centricon filtration at 4°C. Cell lysates and concentrated media were subjected to Western blot analysis with antibody against β-actin or HMGB1. All Western blot results are representative of two to three experiments. C, D, The neuron–glia cultures were treated with vehicle, 400 or 500 ng/ml endotoxin-free recombinant HMGB1 protein. Seven days after the treatment, HMGB1 induced a dose-dependent reduction in DA uptake (C). Representative images confirmed the neuronal loss and dendrite degeneration of DA neurons after HMGB1 treatment (D). E, F, Neutralization of HMGB1 rescued DA neurons from chronic degeneration. During or 48 h after the addition of 10 ng/ml LPS, 0.5 μ
m
MPP+, or 10 n
m
rotenone, neuron–glia cultures were treated with rabbit IgG isotype antibody or anti-HMGB1 antibody. Seven days after the initial treatment, cultures were assayed for DA uptake (E) and cell count of TH-IR neurons (F). The results are the mean ± SEM of three to four experiments performed in triplicate. *p < 0.05 compared with the corresponding LPS-, MPP+-, or rotenone-treated cultures. C, Control; L, LPS; R, rotenone; M, MPP+.
Figure 6.
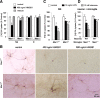
HMGB1 activated microglia and the deficiency in microglial Mac1 attenuated HMGB1-mediated release of inflammatory mediators. A, Neuron–glia cultures were treated for 1–4 d with the vehicle or 400 ng/ml HMGB1 and then were immunostained with the OX-42 antibody. In control cultures, OX-42-IR microglia were small and round. After stimulation with HMGB1, microglia revealed increased immunoreactivity and became larger in size and irregular in shape, indicative of activation. B–D, Neuron–glia cultures prepared from wild-type or _Mac1_−/− mice were treated with vehicle or 400 ng/ml HMGB1 for 12 or 24 h. Levels of TNF-α (B), IL-1β (24 h) (C), and nitrite (an indicator of NO production, 24 h) (D) in the culture medium were measured. Results are means ± SEM of three experiments performed in triplicate. *p < 0.05 compared with the corresponding vehicle-treated control cultures. #p < 0.05 compared with the corresponding HMGB1-treated wild-type cultures.
Figure 7.
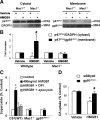
Chronic DA neurodegeneration was attenuated in the absence of microglial Mac1. Neuron–glia cultures (A, B) and neuron-enriched cultures (A) prepared from Mac1+/+ or _Mac1_−/− mice were treated for 7 d with saline, 400 ng/ml HMGB1, or vehicle. A, Attenuation of HMGB1-induced DA neurotoxicity in the absence of microglial Mac1. *p < 0.05 compared with the corresponding saline-treated control cultures. #p < 0.05 compared with the corresponding HMGB1-treated wild-type cultures. B, Representative images of the neuron–glia cultures immunostained for TH (from 3 experiments) displayed greater lesions of DA neurons in the presence of microglial Mac1. C, D, Neuron–glia cultures from wild-type (WT) or _Mac1_−/− mice (C) and reconstituted cultures in which wild-type neuron-enriched cultures were supplemented with either Mac1+/+ or _Mac1_−/− microglia (D) were treated with vehicle, 10 ng/ml LPS, or 10 n
m
rotenone. [3H]DA uptake was determined 7 d later. *p < 0.05 compared with the corresponding vehicle-treated control cultures. #p < 0.05 compared with the corresponding LPS- or rotenone-treated wild-type cultures (C) or reconstituted cultures containing wild-type microglia (D). Note that, in the reconstituted cultures, the deletion of microglial Mac1 rendered DA neurons more resistant to inflammation-mediated neurotoxicity. All quantification results are means ± SEM of three to four experiments performed in triplicate. NG, Neuron–glia cultures; N, neuron-enriched cultures.
Figure 8.
Interactions between HMGB1 and Mac1. A, Primary microglia from rat, _Mac1_−/− mice, or wild-type mice were treated with vehicle or 500 ng/ml HMGB1 at 4°C for 2 h or 37°C for 15 min. Cell lysates were collected in TNE buffer and then immunoprecipitated with antibodies against HMGB1 or Mac1. Separated immunoprecipitates were immunoblotted and probed for Mac1 and, after stripping the membrane, for HMGB1 or vice versa. Anti-HMGB1 antibody coimmunoprecipitated Mac1 in wild-type microglia treated with HMGB1, whereas anti-Mac1 antibody coimmunoprecipitated HMGB1. IP, Immunoprecipitation. B, Crude membrane fractions from _Mac1_−/− or Mac1+/+ microglia were incubated with 1 μg/ml HMGB1 or vehicle at 4°C for 3 h. The Western blotting indicated more HMGB1 bound to Mac1+/+ microglial membranes than bound to _Mac1_−/− microglial membranes. C, Primary microglia from _Mac1_−/− or wild-type mice were treated with vehicle or 500 ng/ml HMGB1 at 37°C for 15 or 30 min. Whole-cell lysates were extracted in RIPA buffer. The activation of NF-κB pathway, as shown by phosphorylation of IKKα/β, degradation of IκB, and phosphorylation of p65, was observed in HMGB1-treated wild-type microglia, but such activation was blunted in HMGB1-treated _Mac1_−/− microglia. D, The ratios of densitometry values of examined proteins in C were normalized to each responsive control. *p < 0.05 compared with the corresponding vehicle-treated controls. E, F, Nuclear translocation of p65 was observed in rat microglia treated with HMGB1 (500 ng/ml) for 15–30 min. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. All panels are representative of two to three independent experiments.
Figure 9.
HMGB1 activated the Mac1–NADPH oxidase signaling axis leading to microglial activation. A, Mac1 was required for HMGB1-mediated p47phox translocation to cell membrane. Primary microglia-enriched cultures prepared from _Mac1_−/− mice or wild-type mice were treated with vehicle or 500 ng/ml HMGB1 at 37°C for 15 min. Subcellular fractions were isolated to perform Western blotting analysis for p47phox levels in membrane and cytosolic fractions of microglia. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) and gp91phox were used as internal control of cytosolic and membrane fractions, respectively. B, The ratio of densitometry values of cytosolic p47phox to GADPH and membrane p47phox to gp91phox from two independent experiments was normalized to each respective vehicle-treated control. C, Primary microglia from _Mac1_−/−, gp91 _PHOX_−/−, or wild-type (WT) mice were treated with vehicle or 500 ng/ml HMGB1 at 37°C for 15 min. The production of extracellular superoxide was detected by SOD-inhibitable reduction of WST-1. The production of extracellular superoxide was detected only in HMGB1-treated wild-type microglia and was inhibited by the cotreatment of the cultures with DPI and apocynin; microglia with deficiency in Mac1 or gp91phox did not cause detectable superoxide release after HMGB1 treatment. D, Primary neuron–glia cultures from gp91 _PHOX_−/− or wild-type mice were stimulated with 400 ng/ml HMGB1. Seven days later, the neurotoxicity was evaluated by [3H]DA uptake assay. Results were expressed as a percentage of the vehicle-treated control cultures and were the mean ± SEM from three to four independent experiments. *p < 0.05 compared with respective vehicle-treated control cultures. #p < 0.05 compared with HMGB1-treated wild-type cultures.
Similar articles
- Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease.
Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA, Ali SF, Zhang J, Block ML, Hong JS. Hu X, et al. J Immunol. 2008 Nov 15;181(10):7194-204. doi: 10.4049/jimmunol.181.10.7194. J Immunol. 2008. PMID: 18981141 Free PMC article. - Microglial MAC1 receptor and PI3K are essential in mediating β-amyloid peptide-induced microglial activation and subsequent neurotoxicity.
Zhang D, Hu X, Qian L, Chen SH, Zhou H, Wilson B, Miller DS, Hong JS. Zhang D, et al. J Neuroinflammation. 2011 Jan 13;8(1):3. doi: 10.1186/1742-2094-8-3. J Neuroinflammation. 2011. PMID: 21232086 Free PMC article. - Activation of neuronal NADPH oxidase NOX2 promotes inflammatory neurodegeneration.
Tu D, Velagapudi R, Gao Y, Hong JS, Zhou H, Gao HM. Tu D, et al. Free Radic Biol Med. 2023 May 1;200:47-58. doi: 10.1016/j.freeradbiomed.2023.03.001. Epub 2023 Mar 2. Free Radic Biol Med. 2023. PMID: 36870375 Free PMC article. - Critical role of the Mac1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration.
Chen SH, Oyarzabal EA, Hong JS. Chen SH, et al. Curr Opin Pharmacol. 2016 Feb;26:54-60. doi: 10.1016/j.coph.2015.10.001. Epub 2015 Oct 26. Curr Opin Pharmacol. 2016. PMID: 26498406 Free PMC article. Review. - Inflammatory neurodegeneration and mechanisms of microglial killing of neurons.
Brown GC, Neher JJ. Brown GC, et al. Mol Neurobiol. 2010 Jun;41(2-3):242-7. doi: 10.1007/s12035-010-8105-9. Epub 2010 Mar 2. Mol Neurobiol. 2010. PMID: 20195798 Review.
Cited by
- Recombinant high-mobility group box 1 protein (HMGB-1) promotes myeloid differentiation primary response protein 88 (Myd88) upregulation in mouse primary cortical neurons.
Li W, Ling HP, You WC, Ji XJ, Tang Y, Zhao JB, Su XF, Hang CH. Li W, et al. Neurol Sci. 2013 Jun;34(6):847-53. doi: 10.1007/s10072-012-1131-9. Epub 2012 Jun 19. Neurol Sci. 2013. PMID: 22710699 - The role of glia in α-synucleinopathies.
Fellner L, Stefanova N. Fellner L, et al. Mol Neurobiol. 2013 Apr;47(2):575-86. doi: 10.1007/s12035-012-8340-3. Epub 2012 Sep 2. Mol Neurobiol. 2013. PMID: 22941028 Free PMC article. Review. - Translocator protein 18 kDa negatively regulates inflammation in microglia.
Bae KR, Shim HJ, Balu D, Kim SR, Yu SW. Bae KR, et al. J Neuroimmune Pharmacol. 2014 Jun;9(3):424-37. doi: 10.1007/s11481-014-9540-6. Epub 2014 Apr 1. J Neuroimmune Pharmacol. 2014. PMID: 24687172 - Pathways to neurodegeneration: mechanistic insights from GWAS in Alzheimer's disease, Parkinson's disease, and related disorders.
Ramanan VK, Saykin AJ. Ramanan VK, et al. Am J Neurodegener Dis. 2013 Sep 18;2(3):145-75. Am J Neurodegener Dis. 2013. PMID: 24093081 Free PMC article. Review. - High expression of the HMGB1-TLR4 axis and its downstream signaling factors in patients with Parkinson's disease and the relationship of pathological staging.
Yang Y, Han C, Guo L, Guan Q. Yang Y, et al. Brain Behav. 2018 Mar 25;8(4):e00948. doi: 10.1002/brb3.948. eCollection 2018 Apr. Brain Behav. 2018. PMID: 29670828 Free PMC article.
References
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. - PubMed
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. - PubMed
- Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases