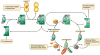Role of Rab GTPases in membrane traffic and cell physiology - PubMed (original) (raw)
Review
Role of Rab GTPases in membrane traffic and cell physiology
Alex H Hutagalung et al. Physiol Rev. 2011 Jan.
Abstract
Intracellular membrane traffic defines a complex network of pathways that connects many of the membrane-bound organelles of eukaryotic cells. Although each pathway is governed by its own set of factors, they all contain Rab GTPases that serve as master regulators. In this review, we discuss how Rabs can regulate virtually all steps of membrane traffic from the formation of the transport vesicle at the donor membrane to its fusion at the target membrane. Some of the many regulatory functions performed by Rabs include interacting with diverse effector proteins that select cargo, promoting vesicle movement, and verifying the correct site of fusion. We describe cascade mechanisms that may define directionality in traffic and ensure that different Rabs do not overlap in the pathways that they regulate. Throughout this review we highlight how Rab dysfunction leads to a variety of disease states ranging from infectious diseases to cancer.
Conflict of interest statement
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
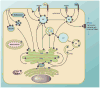
FIG. 1
The intracellular localization of Rabs. A typical cell showing the intracellular localization and associated vesicle transport pathway(s) of several Rab GTPases. Rab1 regulates ER-Golgi traffic while Rab2 is involved in recycling, or retrograde traffic, from Golgi and the ERGIC back to the ER. Rab6 regulates intra-Golgi traffic. Several Rabs including Rab8, -10, and -14 regulate biosynthetic traffic from the _trans_-Golgi network (TGN) to the plasma membrane. The glucose transporter GLUT4 is found in vesicles that use these Rabs to reach the plasma membrane. Several secretory vesicles and granules use Rab3, -26, -27, and -37 to exocytose their cargo. Rab27 is well-studied in the melanosome transport that also relies on Rabs 32 and 38. There are numerous Rabs associated with endosomal traffic, and the most active site of localization is the early endosome. Most early endocytic steps rely on Rab5, which mediates fusion of endocytic vesicles to form the early endosome. Traffic can be directed towards the lysosome for degradation, which relies on action of Rab7, or to various recycling compartments to return factors to the plasma membrane. Rab15 directs membrane traffic from the early endosome to the recycling endosome. Rab4 and Rab11 regulate fast and slow endocytic recycling, respectively. Specialized Rab functions include Rab18-mediated regulation of lipid droplets, intracellular lipid storage sites. Rab24 and Rab33 mediate formation of the preautophagosomal structure that engulfs cellular components to form the autophagosome that is subsequently targeted to the lysosome/vacuole. Rab21 and Rab25 regulate transport of integrins to control cell adhesion and cytokinesis. Rab13 directs traffic to and regulates formation of tight junctions in polarized epithelial cells. Tight junctions define the boundary between the apical and basolateral regions of the polarized cell. Mutations in the mouse Rab23 gene lead to a severe developmental defect, open brain, because Rab23 acts downstream to negatively regulate Sonic hedgehog (Shh) signaling during dorsoventral development of the mouse spinal cord. It potentially interacts with the transcription factors activated by the Shh pathway. Rab40 also acts in a signaling pathway; it recruits components of the ubiquitination machinery to regulate Wnt signaling. There are several poorly characterized Rabs, such as Rab35. It controls plasma membrane recycling of an essential factor in cytokinesis. Rab34 and Rab39 are found on the Golgi, but it is unclear what role they play. AP, autophagosome; ERGIC, ER-Golgi intermediate compartment; ER, endoplasmic reticulum; EE, early endosome; LD, lipid droplet; LE, late endosome (multivesicular body); L/V, lysosome/vacuole; PAS, preautophagosomal structure; RE, recycling endosome; SV, secretory vesicle/granule.
FIG. 2
The Rab cycle. The newly synthesized Rab protein associates with Rab escort protein (REP) that directs it to Rab geranylgeranyl transferase (RabGGT) to receive its prenyl tails (red wavy lines). REP delivers the Rab to its target membrane. Throughout this process, the Rab is GDP-bound. A guanine nucleotide exchange factor (GEF) catalyzes exchange of GDP for GTP to activate the Rab. The GTP-bound Rab interacts with effector proteins that mediate membrane traffic in the pathway regulated by its associated Rab. The Rab then interacts with its associated GTPase activating protein (GAP) that catalyzes hydrolysis of GTP to GDP by the Rab. The Rab is then removed from the membrane by guanine nucleotide dissociation inhibitor (GDI) in preparation for the next cycle. The insertion of the Rab into the target membrane is mediated by a GDI dissociation factor (GDF) that releases the Rab from GDI. Loss-of-function mutations at each of the above steps produce disease phenotypes as indicated by the red text boxes.
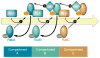
FIG. 3
Rab effectors. Rabs perform their regulatory function by recruiting a variety of effectors to mediate different functions in membrane transport. These functions are as follows: 1) cargo selection/budding/coat formation, 2) vesicle transport, 3) vesicle uncoating/tethering, and 4) vesicle fusion. Below each function are examples of Rab effectors that perform said function. Mutations in Rab effectors also lead to disease phenotypes: Griscelli Syndrome is caused by mutations in either Rab27A, the Rab27A effector protein melanophilin, or myosin VA, while congenital disorders of glycosylation and spondyloepiphyseal dysplasia tarda (SEDT) are caused by mutations in several COG subunits (COG1, COG7, and COG8) and the TRAPP subunit Trs20, respectively.
FIG. 4
The Rab GEF and GAP cascade. Once RabA inserts into its target membrane, it is activated by its respective GEF (step 1). Activated RabA recruits the GEF for the downstream Rab in the pathway RabB (step 2). GTP-bound RabB performs two functions: it recruits the GAP to inactivate RabA (step 3) as well as the GEF for the downstream Rab, RabC (step 4). Activated RabC now recruits the GAP that inactivates RabB (step 5). The concomitant action of GAPs and GEFs ensures the boundaries of each membrane compartment, determined by the actions of their associated Rab, are well-defined.
Similar articles
- Rab proteins: the key regulators of intracellular vesicle transport.
Bhuin T, Roy JK. Bhuin T, et al. Exp Cell Res. 2014 Oct 15;328(1):1-19. doi: 10.1016/j.yexcr.2014.07.027. Epub 2014 Aug 1. Exp Cell Res. 2014. PMID: 25088255 Review. - Toward a comprehensive map of the effectors of rab GTPases.
Gillingham AK, Sinka R, Torres IL, Lilley KS, Munro S. Gillingham AK, et al. Dev Cell. 2014 Nov 10;31(3):358-373. doi: 10.1016/j.devcel.2014.10.007. Epub 2014 Nov 10. Dev Cell. 2014. PMID: 25453831 Free PMC article. - Oligomerization of rab/effector complexes in the regulation of vesicle trafficking.
Khan AR. Khan AR. Prog Mol Biol Transl Sci. 2013;117:579-614. doi: 10.1016/B978-0-12-386931-9.00021-0. Prog Mol Biol Transl Sci. 2013. PMID: 23663983 Review. - Rab GTPases as coordinators of vesicle traffic.
Stenmark H. Stenmark H. Nat Rev Mol Cell Biol. 2009 Aug;10(8):513-25. doi: 10.1038/nrm2728. Epub 2009 Jul 15. Nat Rev Mol Cell Biol. 2009. PMID: 19603039 Review. - Ypt/Rab GTPases: principles learned from yeast.
Lipatova Z, Hain AU, Nazarko VY, Segev N. Lipatova Z, et al. Crit Rev Biochem Mol Biol. 2015;50(3):203-11. doi: 10.3109/10409238.2015.1014023. Epub 2015 Feb 23. Crit Rev Biochem Mol Biol. 2015. PMID: 25702751 Free PMC article. Review.
Cited by
- Alpha-synuclein and intracellular trafficking: impact on the spreading of Parkinson's disease pathology.
Eisbach SE, Outeiro TF. Eisbach SE, et al. J Mol Med (Berl). 2013 Jun;91(6):693-703. doi: 10.1007/s00109-013-1038-9. Epub 2013 Apr 25. J Mol Med (Berl). 2013. PMID: 23616088 Review. - Rab family of small GTPases: an updated view on their regulation and functions.
Homma Y, Hiragi S, Fukuda M. Homma Y, et al. FEBS J. 2021 Jan;288(1):36-55. doi: 10.1111/febs.15453. Epub 2020 Jul 1. FEBS J. 2021. PMID: 32542850 Free PMC article. Review. - The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis.
Onnis A, Finetti F, Patrussi L, Gottardo M, Cassioli C, Spanò S, Baldari CT. Onnis A, et al. Cell Death Differ. 2015 Oct;22(10):1687-99. doi: 10.1038/cdd.2015.17. Epub 2015 Mar 13. Cell Death Differ. 2015. PMID: 26021297 Free PMC article. - Rab6a/a' are important Golgi regulators of pro-inflammatory TNF secretion in macrophages.
Micaroni M, Stanley AC, Khromykh T, Venturato J, Wong CX, Lim JP, Marsh BJ, Storrie B, Gleeson PA, Stow JL. Micaroni M, et al. PLoS One. 2013;8(2):e57034. doi: 10.1371/journal.pone.0057034. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437303 Free PMC article. - The N-terminal Leu-Pro-Gln sequence of Rab34 is required for ciliogenesis in hTERT-RPE1 cells.
Oguchi ME, Homma Y, Fukuda M. Oguchi ME, et al. Small GTPases. 2022 Jan;13(1):77-83. doi: 10.1080/21541248.2021.1894910. Epub 2021 Apr 16. Small GTPases. 2022. PMID: 33860735 Free PMC article.
References
- Ali B, Seabra M. Targeting of Rab GTPases to cellular membranes. Biochem Soc Trans. 2005;33:652–656. - PubMed
- Aligianis I, Johnson C, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina E, Morgan N, Tee L, Morton J, Ainsworth J, Horn D, Rosser E, Cole T, Stolte-Dijkstra I, Fieggen K, Clayton-Smith J, Mégarbané A, Shield J, Newbury-Ecob R, Dobyns W, Graham JJ, Kjaer K, Warburg M, Bond J, Trembath R, Harris L, Takai Y, Mundlos S, Tannahill D, Woods C, Maher E. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet. 2005;37:221–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM035370/GM/NIGMS NIH HHS/United States
- GM-82861/GM/NIGMS NIH HHS/United States
- R01 GM082861/GM/NIGMS NIH HHS/United States
- GM-35370/GM/NIGMS NIH HHS/United States
- R01 GM035370/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases