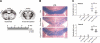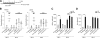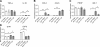Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model - PubMed (original) (raw)
Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model
Hye Jung Kim et al. FASEB J. 2011 May.
Abstract
Fingolimod (FTY720) is a sphingosine 1-phosphate (S1P) receptor modulator that regulates lymphocyte trafficking and exerts pleiotropic actions on oligodendrocytes (OLGs) and other neural cells. The purpose of this study was to investigate the role of S1P receptors in a non-T-cell model of demyelination, the cuprizone (cupr) model in C57BL/6 mice. Treatment with FTY720 (1 mg/kg) led to attenuated injury to OLGs, myelin, and axons in the corpus callosum (percentage of myelinated fibers was 44.7% in cupr-water and 63% in cupr-FTY720). Reactive astrogliosis and microgliosis were ameliorated when FTY720 was given from d 1, but astrogliosis was augmented when FTY720 was given from wk 4-9. FTY720 did not promote remyelination in this model. The protective effect of FTY720 was associated with decreased interleukin-1β and CCL2 transcripts in the corpus callosum, as well as altered S1P1 expression. Targeted deletion of S1P1 in OLG lineage cells did not lead to obvious clinical phenotype, but resulted in subtle abnormalities in myelin and an increased susceptibility to cupr-induced demyelination. We conclude that S1P receptors expressed by neuroglia are involved in regulating the response to injury, and CNS effects of FTY720 could contribute to its favorable therapeutic response in multiple sclerosis.
Figures
Figure 1.
Amelioration of cupr-induced demyelination by FTY720. A) Levels of brain sections (septostriatal and rostral diencephalon) sampled for LFB staining, areas analyzed, and treatment schedule to induce demyelination. Arrows delineate medial and lateral corpus callosum; boxed area delineates center of medial corpus callosum. B) Examples of LFB-stained medial corpus callosum. Scale bar = 200 μm. C) Summary of demyelination scores in the corpus callosum. Data were from n = 3 for control (ctrl) group; n = 5 each for NF-water and NF-FTY720 groups; n = 8–9 for cupr-water and cupr-FTY720 groups. Horizontal bars indicate median values. Ctrl, C57BL/6 mice fed normal diet without gavage; NF, normal food diet; cupr, cuprizone treatment. Both medial and lateral parts of the corpus callosum were similarly affected. *P < 0.01; Mann-Whitney test.
Figure 2.
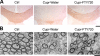
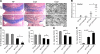
Immunohistochemical and ultrastructural assessment of cupr-induced demyelination in the corpus callosum. A) Images illustrating the MBP immunoreactivity in brain sections from animals in control (ctrl), cupr-water, and cupr-FTY720 treatment groups. B) Representative electron micrographs showing demyelination in the corpus callosum in sections from animals in control, cupr-water, and cupr-FTY720 groups. Scale bars = 200 μm (A); 1 μm (B).
Figure 3.
Effect of FTY720 on OLGs and OPCs in the cupr model. A, C, E, G) Representative images of stained sections. B, F, H) Data summary (_n_=4–5 each). A, B) OLGs. *P < 0.001; **P < 0.013. C) TUNEL labeling. Arrows indicate apoptotic nuclei (TUNEL+DAPI+). D) Western blots of corpus callosum homogenates demonstrating the effect of FTY720 treatment on AIF protein levels (_n_=2). E, F) Late OPCs. Arrows indicate examples of Nkx2.2strong cells. *P < 0.02; **P < 0.04. G, H) Proliferating OPCs. Arrows indicate examples of NG2+PCNA+ cells. *P < 0.01; **P < 0.0003. C, D) Data from cupr-fed animals treated with water or FTY720 for 3 wk; the rest are data from treatment schedule outlined in Fig. 1. Scale bars = 50 μm.
Figure 4.
Decreased accumulation of astrocytes (GFAP+) and microglia/ macrophages (Iba1+) in brain sections from FTY720-treated cupr-fed animals. A, B) Images of brain sections showing the GFAP (green; A) and Iba1 (red; B) immunoreactivity. Nuclei were counterstained with DAPI (blue). Scale bars = 50 μm. C, D) Quantitative analysis of astrogliosis (C) and microgliosis (D) (integrated intensity). Data were normalized to values from control group (ctrl; NF-water) in each set (_n_=4 or 5 each). *P < 0.03 (C); *P < 0.016 (D).
Figure 5.
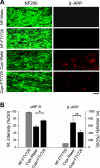
Attenuation of axonal degeneration by FTY720 in the cupr model. A) Representative images showing immunoreactivity against pNF-H (green) and β-amyloid precursor protein (β-APP; red) in the corpus callosum. Scale bar = 10 μm. B) Quantitative analysis of pNF-H and β-APP immunoreactivity (integrated intensity). Data were normalized to values from control group (ctrl; NF-water; _n_=4–5/group). *P < 0.004; **P < 0.02.
Figure 6.
Effect of FTY720 on remyelination and astrogliosis (recovery phase). A) Treatment schedule to induce demyelination and remyelination in these studies. B) Lack of effect of FTY720 on remyelination in the corpus callosum. Presence of demyelination at wk 6 was confirmed for NF vs. cupr without gavage (_n_=5 or 6). For mice sacrificed at wk 9, n = 6–9 for NF-water and NF-FTY720 groups; n = 11–15 for cupr-water and cupr-FTY720 (0.3 and 1 mg/kg) groups. *P<0.001. C) Cupr-induced astrogliosis was augmented by FTY720 treatment when given from wk 4 to 9; n values similar to B. *P < 0.05; **P < 0.01. D) FTY720 had no effect on cupr-induced accumulation of microglia/macrophages during remyelination; n values similar to B. *P<0.03.
Figure 7.
Effect of FTY720 on the expression of selected cytokines, chemokines, growth factors, and S1P receptors in the corpus callosum during toxic demyelination. At least 3 independent experiments were done with each condition in triplicates. Because of extremely low levels of expression of IL-1β, CCL2, CCL5, and IGF-1 under control conditions, data were normalized to GAPDH mRNA (r_=2−Δ_CT) instead of the analysis by comparative method 2−ΔΔ_CT_. A) TNF-α and IL-1β transcripts. *P < 0.01; **P < 0.0001. B) CCL2 and CCL5 transcripts. *P < 0.002; **P < 0.0002. C) PDGF and IGF-1 transcripts. *P < 0.0005. D) S1P1 and S1P5 transcripts. *P < 0.002 for cupr-water vs. NF-water; *P < 0.01 for cupr-water vs. cupr-FTY720; **P < 0.0004.
Figure 8.
Targeted deletion of S1P1 in OLG lineage cells increases the susceptibility to cupr-induced demyelination. A) Examples of LFB-stained septostriatal sections (color) and electron micrographs (grayscale) of the corpus callosum from WT and _S1P1_-CKO mice after 3 wk of cupr diet. Scale bars = 200 μm (LFB); 2 μm (EM). B) Demyelination scores in the medial corpus callosum (_n_=4 or 5 each for WT-NF and _S1P1_-CKO-NF; _n_=7 each for WT-cupr and _S1P1_-CKO-cupr). *P < 0.005; Mann-Whitney test. C) Cupr-induced loss of myelinated axons at 3 wk (EM analysis; _n_=3 each). *P < 0.03. D) Data summary on the number of OLGs (CC1+ cells; _n_=6–7 each). *P < 0.05. E) Cupr-induced axonal damage (decreased pNF-H integrated intensity; _n_=5 each). *P < 0.04. F) Cupr-induced axonal damage (increased β-APP integrated intensity; _n_=5 each). *P < 0.007. Data were normalized to the integrated intensity of NF-water.
Similar articles
- The sphingosine 1-phosphate receptor agonist FTY720 is neuroprotective after cuprizone-induced CNS demyelination.
Slowik A, Schmidt T, Beyer C, Amor S, Clarner T, Kipp M. Slowik A, et al. Br J Pharmacol. 2015 Jan;172(1):80-92. doi: 10.1111/bph.12938. Epub 2014 Dec 15. Br J Pharmacol. 2015. PMID: 25220526 Free PMC article. - Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo.
Hu Y, Lee X, Ji B, Guckian K, Apicco D, Pepinsky RB, Miller RH, Mi S. Hu Y, et al. Mol Cell Neurosci. 2011 Sep;48(1):72-81. doi: 10.1016/j.mcn.2011.06.007. Epub 2011 Jun 24. Mol Cell Neurosci. 2011. PMID: 21740973 - Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices.
Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. Miron VE, et al. Am J Pathol. 2010 Jun;176(6):2682-94. doi: 10.2353/ajpath.2010.091234. Epub 2010 Apr 22. Am J Pathol. 2010. PMID: 20413685 Free PMC article. - FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system.
Brinkmann V. Brinkmann V. Br J Pharmacol. 2009 Nov;158(5):1173-82. doi: 10.1111/j.1476-5381.2009.00451.x. Epub 2009 Oct 8. Br J Pharmacol. 2009. PMID: 19814729 Free PMC article. Review. - FTY720 on the way from the base camp to the summit of the mountain: relevance for remyelination.
Kipp M, Amor S. Kipp M, et al. Mult Scler. 2012 Mar;18(3):258-63. doi: 10.1177/1352458512438723. Mult Scler. 2012. PMID: 22383435 Review.
Cited by
- Lysophospholipids and their receptors in the central nervous system.
Choi JW, Chun J. Choi JW, et al. Biochim Biophys Acta. 2013 Jan;1831(1):20-32. doi: 10.1016/j.bbalip.2012.07.015. Epub 2012 Jul 31. Biochim Biophys Acta. 2013. PMID: 22884303 Free PMC article. Review. - A2 B Adenosine Receptors and Sphingosine 1-Phosphate Signaling Cross-Talk in Oligodendrogliogenesis.
Coppi E, Cencetti F, Cherchi F, Venturini M, Donati C, Bruni P, Pedata F, Pugliese AM. Coppi E, et al. Front Neurosci. 2021 May 26;15:677988. doi: 10.3389/fnins.2021.677988. eCollection 2021. Front Neurosci. 2021. PMID: 34135730 Free PMC article. Review. - Effect of Fingolimod on Neural Stem Cells: A Novel Mechanism and Broadened Application for Neural Repair.
Zhang Y, Li X, Ciric B, Ma CG, Gran B, Rostami A, Zhang GX. Zhang Y, et al. Mol Ther. 2017 Feb 1;25(2):401-415. doi: 10.1016/j.ymthe.2016.12.008. Epub 2016 Dec 28. Mol Ther. 2017. PMID: 28153091 Free PMC article. - Sphingosine-1-Phosphate: Its Pharmacological Regulation and the Treatment of Multiple Sclerosis: A Review Article.
Cohan S, Lucassen E, Smoot K, Brink J, Chen C. Cohan S, et al. Biomedicines. 2020 Jul 18;8(7):227. doi: 10.3390/biomedicines8070227. Biomedicines. 2020. PMID: 32708516 Free PMC article. Review. - Cuprizone-induced demyelination in mice: age-related vulnerability and exploratory behavior deficit.
Wang H, Li C, Wang H, Mei F, Liu Z, Shen HY, Xiao L. Wang H, et al. Neurosci Bull. 2013 Apr;29(2):251-9. doi: 10.1007/s12264-013-1323-1. Epub 2013 Apr 5. Neurosci Bull. 2013. PMID: 23558591 Free PMC article.
References
- Spiegel S., Milstien S. (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Mol. Cell. Biol. 4, 397–407 - PubMed
- Brinkmann V., Davis M. D., Heise C. E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., Foster C. A., Zollinger M., Lynch K. R. (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277, 21453–21457 - PubMed
- Fujino M., Funeshima N., Kitazawa Y., Kimura H., Amemiya H., Suzuki S., Li X. K. (2003) Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J. Pharmacol. Exp. Ther. 305, 70–77 - PubMed
- Webb M., Tham C. S., Lin F. F., Lariosa-Willingham K., Yu N., Hale J., Mandala S., Chun J., Rao T. S. (2004) Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J. Neuroimmunol. 153, 108–121 - PubMed
- Balatoni B., Storch M. K., Swoboda E. M., Schonborn V., Koziel A., Lambrou G. N., Hiestand P. C., Weissert R., Foster C. A. (2007) FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain. Res. Bull. 74, 307–316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources