White matter changes and word finding failures with increasing age - PubMed (original) (raw)
White matter changes and word finding failures with increasing age
Emmanuel A Stamatakis et al. PLoS One. 2011.
Abstract
Background: Increasing life expectancy necessitates the better understanding of the neurophysiological underpinnings of age-related cognitive changes. The majority of research examining structural-cognitive relationships in aging focuses on the role of age-related changes to grey matter integrity. In the current study, we examined the relationship between age-related changes in white matter and language production. More specifically, we concentrated on word-finding failures, which increase with age.
Methodology/principal findings: We used Diffusion tensor MRI (a technique used to image, in vivo, the diffusion of water molecules in brain tissue) to relate white matter integrity to measures of successful and unsuccessful picture naming. Diffusion tensor images were used to calculate Fractional Anisotropy (FA) images. FA is considered to be a measure of white matter organization/integrity. FA images were related to measures of successful picture naming and to word finding failures using voxel-based linear regression analyses. Successful naming rates correlated positively with white matter integrity across a broad range of regions implicated in language production. However, word finding failure rates correlated negatively with a more restricted region in the posterior aspect of superior longitudinal fasciculus.
Conclusions/significance: The use of DTI-MRI provides evidence for the relationship between age-related white matter changes in specific language regions and word finding failures in old age.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
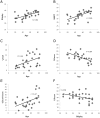
Figure 1. Relationships between background measures, scores from the TOT task and age.
The plots show the relationship between: a) age and Shipley scores (r = .62, p<.001), b) age and NART scores (r = .60, p<.001), c) age and TOT rates (r = .44, p<.01), d) age and Know rates (r = −.64, p<.001), e) age and Don't know rates (r = .47, p<.05) and f) Know rates and Shipley scores (r = −.39, p<.05).
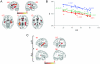
Figure 2. Fractional Anisotropy by age and hemisphere: a) Age-related reductions in FA are shown superimposed on a T1-weighted spatially normalized brain scan.
Left side of the axial slices corresponds to left hemisphere. Color bar indicates range of t-scores. b) FA values obtained from all participants are shown plotted as a function of age. Mean FA values were obtained from 10 mm diameter spheres centered at the peaks of the three most significant voxels resulting from the whole brain SPM analysis. (i) −36 −35 −1 (r = −.87, p<.001), (ii) −47 −45 31 (r = −.83, p<.001) (iii) −2 6 −1 (r = −.86, p<.001). c) Cross-hemispheric FA comparisons are shown superimposed on a T1-weighted spatially normalized brain scan. Color bar indicates range of t-scores, with higher values reflecting greater asymmetry.
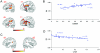
Figure 3. Fractional anisotropy and performance in the TOT task.
a) Correlations between proportion Know responses from the TOT task and FA are shown superimposed on a T1-weighted spatially normalized brain scan. Color bar indicates range of t-scores. b) Mean FA values for each participant, obtained at the statistical peak of −42 −46 6 are shown plotted against proportion Know responses (r = −.75, p<.001). Mean FA values from each participant were obtained from a 10 mm diameter sphere centered at the peak of the statistically significant cluster. c) FA correlations between proportion TOTs and FA are shown superimposed on a T1-weighted spatially normalized brain scan. Color bar indicates range of t-scores. d) Mean FA values for each participant, obtained at the statistical peak of 41 −48 19 are shown plotted against proportion TOTs (r = −.72, p<.001). Mean FA values from each participant were obtained from a 10 mm diameter sphere centered at the peak of the statistically significant cluster.
Similar articles
- White matter abnormalities in first-episode schizophrenia: a combined structural MRI and DTI study.
Chan WY, Yang GL, Chia MY, Lau IY, Sitoh YY, Nowinski WL, Sim K. Chan WY, et al. Schizophr Res. 2010 Jun;119(1-3):52-60. doi: 10.1016/j.schres.2009.12.012. Epub 2010 Jan 6. Schizophr Res. 2010. PMID: 20056394 - Gray and white matter asymmetries in healthy individuals aged 21-29 years: a voxel-based morphometry and diffusion tensor imaging study.
Takao H, Abe O, Yamasue H, Aoki S, Sasaki H, Kasai K, Yoshioka N, Ohtomo K. Takao H, et al. Hum Brain Mapp. 2011 Oct;32(10):1762-73. doi: 10.1002/hbm.21145. Epub 2010 Sep 30. Hum Brain Mapp. 2011. PMID: 20886579 Free PMC article. - Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study.
Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. Teipel SJ, et al. J Alzheimers Dis. 2010;22(2):507-22. doi: 10.3233/JAD-2010-100234. J Alzheimers Dis. 2010. PMID: 20847446 - Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment.
Stebbins GT, Murphy CM. Stebbins GT, et al. Behav Neurol. 2009;21(1):39-49. doi: 10.3233/BEN-2009-0234. Behav Neurol. 2009. PMID: 19847044 Free PMC article. Review. - Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking.
Sullivan EV, Rohlfing T, Pfefferbaum A. Sullivan EV, et al. Dev Neuropsychol. 2010;35(3):233-56. doi: 10.1080/87565641003689556. Dev Neuropsychol. 2010. PMID: 20446131 Free PMC article. Review.
Cited by
- Default Mode Dynamics for Global Functional Integration.
Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA. Vatansever D, et al. J Neurosci. 2015 Nov 18;35(46):15254-62. doi: 10.1523/JNEUROSCI.2135-15.2015. J Neurosci. 2015. PMID: 26586814 Free PMC article. - Cerebral White Matter Mediation of Age-Related Differences in Picture Naming Across Adulthood.
Troutman SBW, Madden DJ, Diaz MT. Troutman SBW, et al. Neurobiol Lang (Camb). 2022 Mar 30;3(2):272-286. doi: 10.1162/nol_a_00065. eCollection 2022 Mar. Neurobiol Lang (Camb). 2022. PMID: 35685085 Free PMC article. - Stronger right hemisphere functional connectivity supports executive aspects of language in older adults.
Gertel VH, Zhang H, Diaz MT. Gertel VH, et al. Brain Lang. 2020 Jul;206:104771. doi: 10.1016/j.bandl.2020.104771. Epub 2020 Apr 11. Brain Lang. 2020. PMID: 32289553 Free PMC article. - Differences in Diffusion Tensor Imaging White Matter Integrity Related to Verbal Fluency Between Young and Old Adults.
Yeske B, Hou J, Adluru N, Nair VA, Prabhakaran V. Yeske B, et al. Front Aging Neurosci. 2021 Nov 22;13:750621. doi: 10.3389/fnagi.2021.750621. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34880746 Free PMC article. - Functional language shift to the right hemisphere in patients with language-eloquent brain tumors.
Krieg SM, Sollmann N, Hauck T, Ille S, Foerschler A, Meyer B, Ringel F. Krieg SM, et al. PLoS One. 2013 Sep 17;8(9):e75403. doi: 10.1371/journal.pone.0075403. eCollection 2013. PLoS One. 2013. PMID: 24069410 Free PMC article.
References
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, et al. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59:156–7. - PubMed
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, et al. A voxel-based morphometric study of aging in 465 normal adult human brains. Neuroimage. 2001;14:21–36. - PubMed
- Sowell ER, Thompson PM, Peterson BS, Welcome SE, Henkenius AL, et al. Mapping Cortical Change Across the Human Lifespan. Nat Neurosci. 2003;6:309–315. - PubMed
- Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, et al. Risk factors for progression of brain atrophy in aging. Six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical