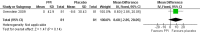Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults - PubMed (original) (raw)
Review
Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults
Anne B Chang et al. Cochrane Database Syst Rev. 2011.
Abstract
Background: Gastroesophageal reflux disease (GORD) is said to be the causative factor in up to 41% of adults with chronic cough. Treatment for GORD includes conservative measures (diet manipulation), pharmaceutical therapy (motility or prokinetic agents, H(2)-antagonist and proton pump inhibitors (PPI)) and fundoplication.
Objectives: To evaluate the efficacy of GORD treatment on chronic cough in children and adults with GORD and prolonged cough that is not related to an underlying respiratory disease, i.e. non-specific chronic cough.
Search strategy: We searched the Cochrane Airways Group Specialised Register, the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, review articles and reference lists of relevant articles. The date of last search was 8 April 2010.
Selection criteria: All randomised controlled trials (RCTs) on GORD treatment for cough in children and adults without primary lung disease.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We contacted study authors for further information.
Main results: We included 19 studies (six paediatric, 13 adults). None of the paediatric studies could be combined for meta-analysis. A single RCT in infants found that PPI (compared to placebo) was not efficacious for cough outcomes (favouring placebo OR 1.61; 95% CI 0.57 to 4.55) but those on PPI had significantly increased adverse events (OR 5.56; 95% CI 1.18 to 26.25) (number needed to treat for harm in four weeks was 11 (95% CI 3 to 232)). In adults, analysis of H(2) antagonist, motility agents and conservative treatment for GORD was not possible (lack of data) and there were no controlled studies of fundoplication. We analysed nine adult studies comparing PPI (two to three months) to placebo for various outcomes in the meta-analysis. Using intention-to-treat, pooled data from studies resulted in no significant difference between treatment and placebo in total resolution of cough (OR 0.46; 95% CI 0.19 to 1.15). Pooled data revealed no overall significant improvement in cough outcomes (end of trial or change in cough scores). We only found significant differences in sensitivity analyses. We found a significant improvement in change of cough scores at end of intervention (two to three months) in those receiving PPI (standardised mean difference -0.41; 95% CI -0.75 to -0.07) using generic inverse variance analysis on cross-over trials. Two studies reported improvement in cough after five days to two weeks of treatment.
Authors' conclusions: PPI is not efficacious for cough associated with GORD symptoms in very young children (including infants) and should not be used for cough outcomes. There is insufficient data in older children to draw any valid conclusions. In adults, there is insufficient evidence to conclude definitely that GORD treatment with PPI is universally beneficial for cough associated with GORD. Clinicians should be cognisant of the period (natural resolution with time) and placebo effect in studies that utilise cough as an outcome measure. Future paediatric and adult studies should be double-blind, randomised controlled and parallel-design, using treatments for at least two months, with validated subjective and objective cough outcomes and include ascertainment of time to respond as well as assessment of acid and/or non-acid reflux.
Conflict of interest statement
Nil.
Figures
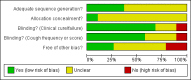
Figure 1
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
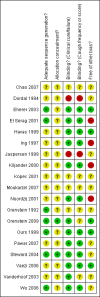
Figure 2
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
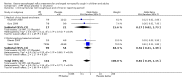
Figure 3
Forest plot of comparison: 2 PPI versus placebo (> 18 years), outcome: 2.1 Clinical failures (still coughing at end of trial or reporting period).
Figure 4
Forest plot of comparison: 3 PPI versus placebo (children), outcome: 3.1 Coughing (% days/week).
Figure 5
Cates plot: In the control group, 25 out of 1000 had a serious adverse event over 4 weeks, compared to 123 (95% CI 29 to 399) out of 1000 for the PPI group
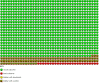
Figure 6
Funnel plot of comparison: 2 PPI versus placebo (> 18 years), outcome: 2.1 Clinical failures (still coughing at end of trial or reporting period).
Analysis 1.1
Comparison 1 Thickened versus unthickened feeds (infants), Outcome 1 Subjects cured (of cough) at end of study.
Analysis 1.2
Comparison 1 Thickened versus unthickened feeds (infants), Outcome 2 Cough frequency.
Analysis 2.1
Comparison 2 PPI versus placebo (children), Outcome 1 Coughing (% days/week).
Analysis 2.2
Comparison 2 PPI versus placebo (children), Outcome 2 Clinical failures (still coughing at end of trial or reporting period).
Analysis 2.3
Comparison 2 PPI versus placebo (children), Outcome 3 Treatment‐emergent serious adverse events.
Analysis 3.1
Comparison 3 PPI versus placebo (> 18 years), Outcome 1 Clinical failures (still coughing at end of trial or reporting period).
Analysis 3.2
Comparison 3 PPI versus placebo (> 18 years), Outcome 2 Mean cough score at end of trial (1st arm cross‐over/parallel‐group trials).
Analysis 3.3
Comparison 3 PPI versus placebo (> 18 years), Outcome 3 Change in cough scores (end‐beginning of intervention ‐ 1st arm cross‐over/parallel‐group trials).
Analysis 3.4
Comparison 3 PPI versus placebo (> 18 years), Outcome 4 Change in cough scores (cross‐over studies; standardised scale).
Analysis 3.5
Comparison 3 PPI versus placebo (> 18 years), Outcome 5 Absolute cough scores (cross‐over studies, standardised scale).
Analysis 3.6
Comparison 3 PPI versus placebo (> 18 years), Outcome 6 Change in cough score after 4 weeks treatment (1st arm cross‐over/parallel‐group trials).
Analysis 3.7
Comparison 3 PPI versus placebo (> 18 years), Outcome 7 Difference in cough scores at week 8 ‐ week 4 (1st arm cross‐over/parallel‐group trials).
Update of
- Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults.
Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Chang AB, et al. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004823. doi: 10.1002/14651858.CD004823.pub3. Cochrane Database Syst Rev. 2006. PMID: 17054216 Updated. Review.
Comment in
- ACP Journal Club. Review: Evidence on PPIs for nonspecific chronic cough in adults with gastroesophageal reflux disease is inconsistent.
Massey BT. Massey BT. Ann Intern Med. 2011 Jun 21;154(12):JC6-9. doi: 10.7326/0003-4819-154-12-201106210-02009. Ann Intern Med. 2011. PMID: 21690587 No abstract available.
Similar articles
- Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults.
Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Chang AB, et al. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004823. doi: 10.1002/14651858.CD004823.pub3. Cochrane Database Syst Rev. 2006. PMID: 17054216 Updated. Review. - Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults.
Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Chang AB, et al. Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004823. doi: 10.1002/14651858.CD004823.pub2. Cochrane Database Syst Rev. 2005. PMID: 15846735 Updated. Review. - Pharmacological treatment of children with gastro-oesophageal reflux.
Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM. Tighe M, et al. Cochrane Database Syst Rev. 2014 Nov 24;2014(11):CD008550. doi: 10.1002/14651858.CD008550.pub2. Cochrane Database Syst Rev. 2014. PMID: 25419906 Free PMC article. Updated. Review. - Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults.
Garg SK, Gurusamy KS. Garg SK, et al. Cochrane Database Syst Rev. 2015 Nov 5;2015(11):CD003243. doi: 10.1002/14651858.CD003243.pub3. Cochrane Database Syst Rev. 2015. PMID: 26544951 Free PMC article. Review. - Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease.
van Pinxteren B, Numans ME, Bonis PA, Lau J. van Pinxteren B, et al. Cochrane Database Syst Rev. 2006 Jul 19;(3):CD002095. doi: 10.1002/14651858.CD002095.pub3. Cochrane Database Syst Rev. 2006. PMID: 16855986 Updated. Review.
Cited by
- Should Acid suppressive therapy improve chronic cough? (Chest 2013;143:605-612).
Oh JH. Oh JH. J Neurogastroenterol Motil. 2013 Jul;19(3):412-4. doi: 10.5056/jnm.2013.19.3.412. Epub 2013 Jul 8. J Neurogastroenterol Motil. 2013. PMID: 23875112 Free PMC article. No abstract available. - Gaviscon® Advance alone versus co-prescription of Gaviscon® Advance and proton pump inhibitors in the treatment of laryngopharyngeal reflux.
Wilkie MD, Fraser HM, Raja H. Wilkie MD, et al. Eur Arch Otorhinolaryngol. 2018 Oct;275(10):2515-2521. doi: 10.1007/s00405-018-5079-0. Epub 2018 Jul 30. Eur Arch Otorhinolaryngol. 2018. PMID: 30062580 - A causal relationship between cough and gastroesophageal reflux disease (GERD) has been established: a pro/con debate.
Kahrilas PJ, Smith JA, Dicpinigaitis PV. Kahrilas PJ, et al. Lung. 2014 Feb;192(1):39-46. doi: 10.1007/s00408-013-9528-7. Epub 2013 Nov 13. Lung. 2014. PMID: 24221340 Free PMC article. - Efficacy and safety of Ojeok-san plus Saengmaek-san for gastroesophageal reflux-induced chronic cough: protocol for a pilot, randomized, double-blind, placebo-controlled trial.
Bhang YH, Kim KI, Kim J, Ahn J, Jung HS, Yang C, Ko SJ, Bu Y, Park JW, Park KS, Jung HJ, Lee JH, Lee BJ. Bhang YH, et al. Trials. 2020 Jan 29;21(1):118. doi: 10.1186/s13063-019-4030-z. Trials. 2020. PMID: 31996267 Free PMC article. - Chronic cough as a disease.
Turner RD, Birring SS. Turner RD, et al. ERJ Open Res. 2024 Nov 18;10(6):00459-2024. doi: 10.1183/23120541.00459-2024. eCollection 2024 Nov. ERJ Open Res. 2024. PMID: 39559449 Free PMC article. Review.
References
References to studies included in this review
- Chao HC, Vandenplas Y. Comparison of the effect of a cornstarch thickened formula and strengthened regular formula on regurgitation, gastric emptying and weight gain in infantile regurgitation. Diseases of the Esophagus 2007;20:155‐60. - PubMed
- Dordal MT, Baltazar MA, Roca I, Marques L, Server MT, Botoy J. Nocturnal spasmodic cough in the infant. Evolution after antireflux treatment [Toux spasmodique nocturne chez l'enfant. Evolution après traitement antireflux]. Allergergie et Immunology 1994;26(2):53‐8. - PubMed
- Eherer AJ, Habermann W, Hammer HF, Kiesler K, Friedrich G, Krejs GJ. Effect of pantoprazole on the course of reflux‐associated laryngitis: a placebo‐controlled double‐blind crossover study. Scandinavian Journal of Gastroenterology 2003;38(5):462‐7. - PubMed
- Serag HB, Lee P, Buchner A, Inadomi JM, Gavin M, McCarthy DM. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo‐controlled trial. American Journal of Gastroenterology 2001;96(4):979‐83. - PubMed
- Havas T, Huang S, Levy M, Abi‐Hanna D, Truskett P, Priestly J, et al. Posterior pharyngolaryngitis: double‐blind randomised placebo‐controlled trial of proton pump inhibitor therapy. Australian Journal of Oto‐Laryngology 1999;3(3):243‐6.
References to studies excluded from this review
- Ahmad I, Batch AJ. Acid reflux management: ENT perspective. Journal of Laryngology & Otology 2004;118(1):25‐30. - PubMed
- Allen CJ, Anvari M. Preoperative symptom evaluation and esophageal acid infusion predict response to laparoscopic Nissen fundoplication in gastroesophageal reflux patients who present with cough. Surgical Endoscopy 2002;16(7):1037‐41. - PubMed
- Allen CJ, Anvari M. Does laparoscopic fundoplication provide long‐term control of gastroesophageal reflux related cough?. Surgical Endoscopy 2004;18(4):633‐7. - PubMed
References to studies awaiting assessment
- Morice AH, Donaldson JE, Fathi HH. The efficacy of dietary intervention in the treatment of reflux cough [Abstract]. American Thoracic Society International Conference. 2008; A897 [#E121].
Additional references
- American Gastroenterological Association. American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996;110(6):1981. - PubMed
- Britt H, Miller GC, Knox S, Charles J, Valenti L, Henderson J, et al. Bettering the Evaluation and Care of Health ‐ A Study of General Practice Activity; 2002. [Australian Institute of Health and Welfare. Report no.: AIHW Cat. No. GE...](Australian Institute of Health and Welfare. Report no.: AIHW Cat. No. GEP‐10 "External link: Australian Institute of Health and Welfare. Report no.: AIHW Cat. No. GEP‐10").
- Chang AB, Newman RG, Carlin J, Phelan PD, Robertson CF. Subjective scoring of cough in children: parent‐completed vs child‐completed diary cards vs an objective method. European Respiratory Journal 1998;11:462‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous