Reduced interhemispheric resting state functional connectivity in cocaine addiction - PubMed (original) (raw)
Reduced interhemispheric resting state functional connectivity in cocaine addiction
Clare Kelly et al. Biol Psychiatry. 2011.
Abstract
Background: Models of cocaine addiction emphasize the role of disrupted frontal circuitry supporting cognitive control processes. However, addiction-related alterations in functional interactions among brain regions, especially between the cerebral hemispheres, are rarely examined directly. Resting-state functional magnetic resonance imaging (fMRI) approaches, which reveal patterns of coherent spontaneous fluctuations in the fMRI signal, offer a means to quantify directly functional interactions between the hemispheres. We examined interhemispheric resting-state functional connectivity (RSFC) in cocaine dependence using a recently validated approach, voxel-mirrored homotopic connectivity.
Methods: We compared interhemispheric RSFC between 25 adults (aged 35.0 ± 8.8) meeting DSM-IV criteria for cocaine dependence within the past 12 months but currently abstaining (>2 weeks) from cocaine and 24 healthy comparisons (35.1 ± 7.5), group-matched on age, sex, education, and employment status.
Results: We observed reduced prefrontal interhemispheric RSFC in cocaine-dependent participants relative to control subjects. Further analyses demonstrated a striking cocaine-dependence-related reduction in interhemispheric RSFC among nodes of the dorsal attention network, comprising bilateral lateral frontal, medial premotor, and posterior parietal areas. Further, within the cocaine-dependent group, RSFC within the dorsal attention network was associated with self-reported attentional lapses.
Conclusions: Our findings provide further evidence of an association between chronic exposure to cocaine and disruptions within large-scale brain circuitry supporting cognitive control. We did not detect group differences in diffusion tensor imaging measures, suggesting that alterations in the brain's functional architecture associated with cocaine exposure can be observed in the absence of detectable abnormalities in the white matter microstructure supporting that architecture.
Copyright © 2011 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Figures
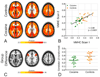
Figure 1. Voxel-Mirrored Homotopic Connectivity (VMHC)
A. Group-level VMHC for the control and cocaine-dependent groups (Z>2.3, cluster-level p<0.05, corrected; images are axial slices at z = 5; 28; and 51). Although there is only one correlation for each pair of homotopic voxels, results are projected on to both hemispheres, to minimize confusion regarding the laterality of the results. B. Cross-scan consistency of mean VMHC values across the IFS area exhibiting significant group differences in the primary VMHC analysis (i.e., the area shown in Figure 1C; controls: r=0.495, p<0.05; cocaine: r=0.587, p<0.01; all participants: r=0.69; p<0.0001). See Supplement for full details of the within-sample replication analysis. C. Inferior frontal sulcus (IFS) area for which the control group exhibited significantly stronger VMHC than the cocaine-dependent group (Z>2.3, cluster-level p<0.05, corrected). D. In recognition of the fact that non-independence in voxel-wise analyses of group differences necessarily provides inflated estimates of effect sizes (–91), the plots show mean VMHC values across the IFS area exhibiting significant group differences in the primary VMHC analysis (shown in B) computed on the basis of the secondary scan (Scan 2) data. The group difference in VMHC for the secondary scan is significant (controls mean Scan 2 VMHC=0.38±0.10, cocaine mean Scan 2 VMHC=0.26±0.09; t(2,38)=4.13, p<0.001).
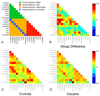
Figure 2. Cocaine-dependent participants exhibit reduced interhemispheric, but not intrahemispheric RSFC within the Dorsal Attention Network (DAN)
We tested for group differences in intrahemispheric (e.g., right IFS and right IPL), heterotopic interhemispheric (e.g., right IFS and left IPL) and homotopic interhemispheric (e.g., right and left IFS) RSFC between all pairs of 16 DAN nodes. Relative to controls, the cocaine-dependent group exhibited reduced homotopic (controls mean=0.45±0.11; cocaine mean=0.39±0.09; t(2,47)=2.66, p<0.05) and heterotopic (controls mean=0.16±0.07; cocaine mean=0.11±0.07; t(2,47)=2.68, p<0.05) interhemispheric RSFC, but not reduced intrahemispheric RSFC (controls left-left mean=0.25±0.07; cocaine left-left mean=0.23±0.08; t(2,47)=0.76, p=0.45; controls right-right mean=0.25±0.1; cocaine right-right mean=0.21±0.08; t(2,47)=1.44, p=0.16). Abbreviations: R: Right; L: Left; antIFS: anterior inferior frontal sulcus; midIFC: middle IFS; postIFS: posterior IFS; preSMA: presupplementary motor area; FEF: frontal eye fields; MT: middle temporal area; IPS: intraparietal sulcus.
Figure 3. ROI-based and voxel-wise brain/behavior relationships
A. Interhemispheric RSFC (i.e., VMHC) within a 4mm-radius sphere centered on the peak of the group difference in VMHC correlated with self-reported cognitive failures, as measured by the Cognitive Failures Questionnaire (CFQ; r=−0.43, _n_=23, p<0.05). Cocaine-dependent participants with the weakest prefrontal interhemispheric RSFC reported experiencing more frequent attentional failures. This relationship remained significant after adjusting for cocaine withdrawal symptoms (as measured by the Cocaine Selective Severity Assessment – CSSA: r=−0.66, p<0.001). B. Voxel-wise analyses revealed relationships between right IFS RSFC and self-reported cognitive failures. Shown in green is the medial/superior lateral premotor area whose RSFC with right IFS exhibited a negative relationship with the CFQ. Cocaine-dependent participants with the weakest RSFC between these two areas reported experiencing more frequent attentional failures. Axial slices (z = 5; 28; and 51) are displayed according to neurological convention (right is right).
Similar articles
- Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction.
Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Hu Y, et al. JAMA Psychiatry. 2015 Jun;72(6):584-92. doi: 10.1001/jamapsychiatry.2015.1. JAMA Psychiatry. 2015. PMID: 25853901 - Aberrant interhemispheric functional and structural connectivity in heroin-dependent individuals.
Qiu YW, Jiang GH, Ma XF, Su HH, Lv XF, Zhuo FZ. Qiu YW, et al. Addict Biol. 2017 Jul;22(4):1057-1067. doi: 10.1111/adb.12387. Epub 2016 Mar 9. Addict Biol. 2017. PMID: 26969418 - Increased interhemispheric resting-state functional connectivity after sleep deprivation: a resting-state fMRI study.
Zhu Y, Feng Z, Xu J, Fu C, Sun J, Yang X, Shi D, Qin W. Zhu Y, et al. Brain Imaging Behav. 2016 Sep;10(3):911-9. doi: 10.1007/s11682-015-9490-5. Brain Imaging Behav. 2016. PMID: 26634366 - Impairments of large-scale functional networks in attention-deficit/hyperactivity disorder: a meta-analysis of resting-state functional connectivity.
Gao Y, Shuai D, Bu X, Hu X, Tang S, Zhang L, Li H, Hu X, Lu L, Gong Q, Huang X. Gao Y, et al. Psychol Med. 2019 Nov;49(15):2475-2485. doi: 10.1017/S003329171900237X. Epub 2019 Sep 10. Psychol Med. 2019. PMID: 31500674 Review. - Reduced homotopic interhemispheric connectivity in psychiatric disorders: evidence for both transdiagnostic and disorder specific features.
Yao S, Kendrick KM. Yao S, et al. Psychoradiology. 2022 Nov 24;2(4):129-145. doi: 10.1093/psyrad/kkac016. eCollection 2022 Dec. Psychoradiology. 2022. PMID: 38665271 Free PMC article. Review.
Cited by
- Insulin Resistance-Associated Interhemispheric Functional Connectivity Alterations in T2DM: A Resting-State fMRI Study.
Xia W, Wang S, Spaeth AM, Rao H, Wang P, Yang Y, Huang R, Cai R, Sun H. Xia W, et al. Biomed Res Int. 2015;2015:719076. doi: 10.1155/2015/719076. Epub 2015 Apr 30. Biomed Res Int. 2015. PMID: 26064945 Free PMC article. - Stochastic dynamic causal modeling of working memory connections in cocaine dependence.
Ma L, Steinberg JL, Hasan KM, Narayana PA, Kramer LA, Moeller FG. Ma L, et al. Hum Brain Mapp. 2014 Mar;35(3):760-78. doi: 10.1002/hbm.22212. Epub 2012 Nov 14. Hum Brain Mapp. 2014. PMID: 23151990 Free PMC article. - Evaluation of altered brain activity in type 2 diabetes using various indices of brain function: A resting-state functional magnetic resonance imaging study.
Zhang G, Liu T, Wei W, Zhang R, Wang H, Wang M. Zhang G, et al. Front Hum Neurosci. 2023 Jan 9;16:1032264. doi: 10.3389/fnhum.2022.1032264. eCollection 2022. Front Hum Neurosci. 2023. PMID: 36699964 Free PMC article. - Removing the influence of group variables in high-dimensional predictive modelling.
Aliverti E, Lum K, Johndrow JE, Dunson DB. Aliverti E, et al. J R Stat Soc Ser A Stat Soc. 2021 Jul;184(3):791-811. doi: 10.1111/rssa.12613. Epub 2021 Apr 15. J R Stat Soc Ser A Stat Soc. 2021. PMID: 35755858 Free PMC article. - Decreased prefrontal lobe interhemispheric functional connectivity in adolescents with internet gaming disorder: a primary study using resting-state FMRI.
Wang Y, Yin Y, Sun YW, Zhou Y, Chen X, Ding WN, Wang W, Li W, Xu JR, Du YS. Wang Y, et al. PLoS One. 2015 Mar 4;10(3):e0118733. doi: 10.1371/journal.pone.0118733. eCollection 2015. PLoS One. 2015. PMID: 25738502 Free PMC article.
References
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. - PubMed
- Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102 Suppl 1:33–43. - PubMed
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. - PubMed
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DA016979/DA/NIDA NIH HHS/United States
- R01 MH081218/MH/NIMH NIH HHS/United States
- 2T32DA007254-16A2/DA/NIDA NIH HHS/United States
- R01MH081218/MH/NIMH NIH HHS/United States
- R03 DA024775/DA/NIDA NIH HHS/United States
- R01 MH081218-03/MH/NIMH NIH HHS/United States
- R01 DA016979-05S1/DA/NIDA NIH HHS/United States
- R01DA016979/DA/NIDA NIH HHS/United States
- R03 DA024775-01/DA/NIDA NIH HHS/United States
- R01MH083246/MH/NIMH NIH HHS/United States
- T32 DA007254/DA/NIDA NIH HHS/United States
- R01 MH083246/MH/NIMH NIH HHS/United States
- R03DA024775/DA/NIDA NIH HHS/United States
- R01 MH083246-03/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous