FUBP3 interacts with FGF9 3' microsatellite and positively regulates FGF9 translation - PubMed (original) (raw)
FUBP3 interacts with FGF9 3' microsatellite and positively regulates FGF9 translation
Bing-Huang Gau et al. Nucleic Acids Res. 2011 May.
Abstract
A TG microsatellite in the 3'-untranslated region (UTR) of FGF9 mRNA has previously been shown to modulate FGF9 expression. In the present study, we investigate the possible interacting protein that binds to FGF9 3'-UTR UG-repeat and study the mechanism underlying this protein-RNA interaction. We first applied RNA pull-down assays and LC-MS analysis to identify proteins associated with this repetitive sequence. Among the identified proteins, FUBP3 specifically bound to the synthetic (UG)(15) oligoribonucleotide as shown by supershift in RNA-EMSA experiments. The endogenous FGF9 protein was upregulated in response to transient overexpression and downregulated after knockdown of FUBP3 in HEK293 cells. As the relative levels of FGF9 mRNA were similar in these two conditions, and the depletion of FUBP3 had no effect on the turn-over rate of FGF9 mRNA, these data suggested that FUBP3 regulates FGF9 expression at the post-transcriptional level. Further examination using ribosome complex pull-down assay showed overexpression of FUBP3 promotes FGF9 expression. In contrast, polyribosome-associated FGF9 mRNA decreased significantly in FUBP3-knockdown HEK293 cells. Finally, reporter assay suggested a synergistic effect of the (UG)-motif with FUBP3 to fine-tune the expression of FGF9. Altogether, results from this study showed the novel RNA-binding property of FUBP3 and the interaction between FUBP3 and FGF9 3'-UTR UG-repeat promoting FGF9 mRNA translation.
© The Author(s) 2011. Published by Oxford University Press.
Figures
Figure 1.
FGF9 3′-UTR RNA structure prediction. (A) Sequence analysis of human FGF9 3′-UTR. The sequence corresponding to the 3′-end of the gene is depicted. The TGA nucleotide sequence marked in bold type corresponds to the translation stop codon. The (GA) and (TG) MS motifs are marked in bold and underlines, and the AU-rich element (ARE) sequence and poly-A signals are marked with double underlines. (B) Representative RNA structure of full-length FGF9 3′-UTR sequence (NM_002010; 613 nt). (C) Enlargement of boxed area in B. Curved lines mark the locations of indicated UG repeats. MFE of predicted structure under each indicated UG repeat in units of kcal/mol.
Figure 2.
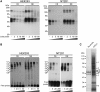
Riboprotein complex formation by UG repeats of FGF9 3′-UTR. (A) UV-cross-linking was conducted using biotinylated (UG)15 oligoribonucleotide and protein extracts (cytoplasmic extract, CE; nuclear extract, NE) prepared from HEK293 (left) and NT2D1 (right) cells. Excess molar ratio of unlabeled cold probe was added as indicated in the competition assay. (B) The same labeled probe, cold probe and protein extracts as in (A), were used in RNA-EMSA and showed similar results in HEK293 (left) and NT2D1 (right) cells. C1–C6: complexes 1–6. (C) (UG)15 binding proteins identified by LC–MS. Proteins pulled down in the absence (left lane) or the presence of riboprobe (UG)15 (right lane) were assayed by silver stain. Differentially expressed bands (marked by 1–8) were excised for protein identification by LC–MS.
Figure 3.
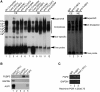
Identification of FUBP3 as UG-repeat binding protein. (A) The RNA–protein complex was detected using specific antibodies as indicated in RNA-EMSA in the protein extracts from NT2D1 (left) and HEK293 (right) cells. CE, cytoplasmic extract; NE, nuclear extract. IgG was used as a control. (B) Pull-down assays were performed using biotinylated (UG)15 riboprobe and _in vitro_-transcribed FGF9 3′-UTR containing (UG)15 repeats in the cytoplasmic extract from HEK293 cells. Specific antibodies to FUBP3, GAPDH and AUF1 were used to detect the pulled-down proteins. H2O and _in vitro_-transcribed GAPDH probe were used as negative controls for pull-down assays. (C) Immunoprecipitated RNA–protein complexes using anti-FUBP3 antibody showed the presence of endogenous FGF9, but not GAPDH mRNA in HEK293 cells (lane 2). Goat normal IgG was used as a control for the immunoprecipitation reaction. The enrichment of FGF9 mRNA precipitated by anti-FUBP3 antibody is shown relative to goat normal IgG. Data were normalized to GAPDH.
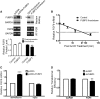
Figure 4.
FUBP3 increased the expression of endogenous FGF9 and reporter proteins containing FGF9 3′-UTR. (A) Representative western blots and RT-PCR results from transient FUBP3 overexpression and shRNA knockdown. Endogenous FUBP3 (filled triangle) and overexpressed FUBP3 (open triangle) were shown in the top panel. Relative mRNA level (RT-PCR; middle panel) and protein amount (ELISA; bottom panel) were shown under each treatments. (B) RNA stability assay showed that knockdown of FUBP3 had no effect on FGF9 turn-over rate. (C) FUBP3 overexpression promotes FGF9 protein synthesis in HEK293 cells. The ribosome complexes were pulled down by 40S ribosomal protein S6 antibody from HEK293 cells overexpressing recombinant FUBP3. The mRNAs bound with ribosome complexes were extracted and analyzed by quantitative RT-PCR. FGF9 mRNA expression level was detected and normalized with the mRNA expression level of GAPDH. The total RNA was also extracted from HEK293 cell with or without FUBP3-overexpression (Right), and the FGF9 mRNA level was detected as an experimental comparison to the results showed in (Left). (D) Relative translational efficiency representation of FGF9 and GAPDH in the polysomal fractions. Ribosome-associated transcripts were measured using quantitative RT-PCR and translational efficiency of FGF9 and GAPDH under FUBP3-knockdown were normalized with the translational efficiency in GFP-knockdown cells. **P < 0.01; ***P < 0.001.
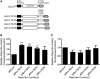
Figure 5.
Synergistic effect of FUBP3 with FGF9 (UG)n repeats. (A) Schematic diagram of reporter constructs containing partial FGF9 3′-UTR with various TG repeats. Reporter activities of various constructs from HEK293 cells overexpressing (B) or knockdown (C) FUBP3. All values were normalized to Renilla luciferase activity produced from a cotransfected control plasmid. Error bars represent standard deviations from three independent transfections. Statistical analyses were conducted by one-way ANOVA. *P < 0.05; **P < 0.01.
Similar articles
- Microsatellite in the 3' untranslated region of human fibroblast growth factor 9 (FGF9) gene exhibits pleiotropic effect on modulating FGF9 protein expression.
Chen TM, Kuo PL, Hsu CH, Tsai SJ, Chen MJ, Lin CW, Sun HS. Chen TM, et al. Hum Mutat. 2007 Jan;28(1):98. doi: 10.1002/humu.9471. Hum Mutat. 2007. PMID: 17154280 - Overexpression of FGF9 in colon cancer cells is mediated by hypoxia-induced translational activation.
Chen TM, Shih YH, Tseng JT, Lai MC, Wu CH, Li YH, Tsai SJ, Sun HS. Chen TM, et al. Nucleic Acids Res. 2014 Mar;42(5):2932-44. doi: 10.1093/nar/gkt1286. Epub 2013 Dec 10. Nucleic Acids Res. 2014. PMID: 24334956 Free PMC article. - AUF1 p42 isoform selectively controls both steady-state and PGE2-induced FGF9 mRNA decay.
Chen TM, Hsu CH, Tsai SJ, Sun HS. Chen TM, et al. Nucleic Acids Res. 2010 Dec;38(22):8061-71. doi: 10.1093/nar/gkq717. Epub 2010 Aug 16. Nucleic Acids Res. 2010. PMID: 20716519 Free PMC article. - Absence of inactivating mutations and deletions in the DMRT1 and FGF9 genes in a large cohort of 46,XY patients with gonadal dysgenesis.
Machado AZ, da Silva TE, Frade Costa EM, Dos Santos MG, Nishi MY, Brito VN, Mendonca BB, Domenice S. Machado AZ, et al. Eur J Med Genet. 2012 Dec;55(12):690-4. doi: 10.1016/j.ejmg.2012.07.012. Epub 2012 Aug 9. Eur J Med Genet. 2012. PMID: 22939835 - The 3'-untranslated region of p21WAF1 mRNA is a composite cis-acting sequence bound by RNA-binding proteins from breast cancer cells, including HuR and poly(C)-binding protein.
Giles KM, Daly JM, Beveridge DJ, Thomson AM, Voon DC, Furneaux HM, Jazayeri JA, Leedman PJ. Giles KM, et al. J Biol Chem. 2003 Jan 31;278(5):2937-46. doi: 10.1074/jbc.M208439200. Epub 2002 Nov 12. J Biol Chem. 2003. PMID: 12431987
Cited by
- Microsatellite polymorphisms associated with human behavioural and psychological phenotypes including a gene-environment interaction.
Bagshaw AT, Horwood LJ, Fergusson DM, Gemmell NJ, Kennedy MA. Bagshaw AT, et al. BMC Med Genet. 2017 Feb 3;18(1):12. doi: 10.1186/s12881-017-0374-y. BMC Med Genet. 2017. PMID: 28158988 Free PMC article. - Functional Mechanisms of Microsatellite DNA in Eukaryotic Genomes.
Bagshaw ATM. Bagshaw ATM. Genome Biol Evol. 2017 Sep 1;9(9):2428-2443. doi: 10.1093/gbe/evx164. Genome Biol Evol. 2017. PMID: 28957459 Free PMC article. Review. - IGF-I 3' untranslated region: strain-specific polymorphisms and motifs regulating IGF-I in osteoblasts.
Smith SS, Kessler CB, Shenoy V, Rosen CJ, Delany AM. Smith SS, et al. Endocrinology. 2013 Jan;154(1):253-62. doi: 10.1210/en.2012-1476. Epub 2012 Nov 26. Endocrinology. 2013. PMID: 23183171 Free PMC article. - The new face of the old molecules: crustin Pm4 and transglutaminase type I serving as rnps down-regulate astakine-mediated hematopoiesis.
Chang YT, Lin CY, Tsai CY, Siva VS, Chu CY, Tsai HJ, Song YL. Chang YT, et al. PLoS One. 2013 Aug 27;8(8):e72793. doi: 10.1371/journal.pone.0072793. eCollection 2013. PLoS One. 2013. PMID: 24013515 Free PMC article. - Transcriptomics-proteomics Integration reveals alternative polyadenylation driving inflammation-related protein translation in patients with diabetic nephropathy.
Zhao T, Zhan D, Qu S, Jiang S, Gan W, Qin W, Zheng C, Cheng F, Lu Y, Liu M, Shi J, Liang H, Wang Y, Qin J, Zen K, Liu Z. Zhao T, et al. J Transl Med. 2023 Feb 6;21(1):86. doi: 10.1186/s12967-023-03934-w. J Transl Med. 2023. PMID: 36747266 Free PMC article.
References
- Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994;371:215–220. - PubMed
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
- Fabregat I, Koch KS, Aoki T, Atkinson AE, Dang H, Amosova O, Fresco JR, Schildkraut CL, Leffert HL. Functional pleiotropy of an intramolecular triplex-forming fragment from the 3′-UTR of the rat Pigr gene. Physiol. Genomics. 2001;5:53–65. - PubMed
- Chiba-Falek O, Nussbaum RL. Effect of allelic variation at the NACP-Rep1 repeat upstream of the alpha-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum. Mol. Genet. 2001;10:3101–3109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous