Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus - PubMed (original) (raw)
Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus
M S Hsu et al. Neuroscience. 2011.
Abstract
Mice deficient in the water channel aquaporin-4 (AQP4) demonstrate increased seizure duration in response to hippocampal stimulation as well as impaired extracellular K+ clearance. However, the expression of AQP4 in the hippocampus is not well described. In this study, we investigated (i) the developmental, laminar and cell-type specificity of AQP4 expression in the hippocampus; (ii) the effect of Kir4.1 deletion on AQP4 expression; and (iii) performed Western blot and RT-PCR analyses. AQP4 immunohistochemistry on coronal sections from wild-type (WT) or Kir4.1-/- mice revealed a developmentally-regulated and laminar-specific pattern, with highest expression in the CA1 stratum lacunosum-moleculare (SLM) and the molecular layer (ML) of the dentate gyrus (DG). AQP4 was colocalized with the glial markers glial fibrillary acidic protein (GFAP) and S100β in the hippocampus, and was also ubiquitously expressed on astrocytic endfeet around blood vessels. No difference in AQP4 immunoreactivity was observed in Kir4.1-/- mice. Electrophysiological and postrecording RT-PCR analyses of individual cells revealed that AQP4 and Kir4.1 were co-expressed in nearly all CA1 astrocytes. In NG2 cells, AQP4 was also expressed at the transcript level. This study is the first to examine subregional AQP4 expression during development of the hippocampus. The strikingly high expression of AQP4 in the CA1 SLM and DG ML identifies these regions as potential sites of astrocytic K+ and H2O regulation. These results begin to delineate the functional capabilities of hippocampal subregions and cell types for K+ and H2O homeostasis, which is critical to excitability and serves as a potential target for modulation in diverse diseases.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
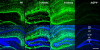
Figure 1. Developmental regulation of AQP4 immunoreactivity in mouse hippocampus
Low-power views of coronal mouse brain sections in P9 vs. 3-week-old vs. 6-week-old mice. While AQP4 immunoreactivity (green) is strongest in the CA1 SLM at P9, AQP4 immunoreactivity at 3 and 6 weeks is dramatically upregulated, particularly in CA1 SLM but also more diffusely throughout the hippocampus. Blood vessels are labeled well at all developmental stages. No specific immunoreactivity is present in sections from adult AQP4-/- mice (right). Nissl counterstains (blue) are shown in the bottom panels for each image. SO=stratum oriens; SP= stratum pyramidale; SR=stratum radiatum; SLM=stratum lacunosum moleculare; ML=molecular layer; DGC=dentate granule cell layer; H=hilus. Scale bar, 300 μm.
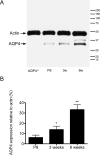
Figure 2. Western blot analysis of hippocampal AQP4 during development
A. Microdissected hippocampal homogenates were isolated from P9, 3-week, and 6-week-old WT mice and 6-week-old AQP4-/- mice and subjected to Western blot analysis for AQP4 and actin. Representative immunoblots demonstrate developmental regulation of AQP4 hippocampal protein levels (lower band, AQP4; upper band, actin loading control). B. Densitometric analysis demonstrates developmental upregulation of AQP4 expression from P9, 3-week, and 6-week samples (expressed as % actin expression) (n=3 per time point). *, p<0.05 compared to 6 weeks; **, p<0.01 compared to P9.
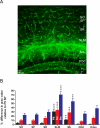
Figure 3. Laminar specificity of hippocampal AQP4 immunoreactivity
A. AQP4 immunoreactivity from 6-week-old mouse hippocampus demonstrates laminar specificity. While blood vessels are labeled well in all laminae, parenchymal AQP4 immunoreactivity is strongest in CA1 stratum lacunosum moleculare (SLM) and dentate gyrus molecular layer (ML) and along the hippocampal fissure separating these laminae. Laminar boundaries are derived from Nissl co-labeling (not shown for clarity). Scale bar, 50 μm. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; DGC, dentate granule cell layer; H, hilus. B. Developmental and laminar-specific quantitation of hippocampal AQP4 immunoreactivity. Values are expressed as % difference in gray value compared with P9 stratum pyramidale (SP) (see Materials and Methods). P9, black bars; 3 weeks, red bars; 6 weeks, blue bars. Higher values indicate more intense immunoreactivity. **, p<0.01 and ***, p<0.001 compared with SP at same time point.
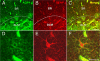
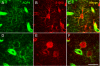
Figure 4. Colocalization of AQP4 and GFAP
Immunoreactivity for AQP4 (A), GFAP (B) and merged (C, F) is shown at the hippocampal CA1 SLM/SR border (dotted line) in a 3-week-old mouse. Note that in the SLM, there is significant colocalization of GFAP and AQP4 on cells with the morphology of bona fide astrocytes, whereas in the SR, GFAP-labeled astrocytes are seen that are AQP4-negative. AQP4 labels blood vessels in both layers. Higher-power images (D-F) demonstrate AQP4 and GFAP colocalization on an astrocyte in CA1 SR from a 6-week-old mouse. Scale bars, 50 μm (top), 20 μm (bottom).
Figure 5. Colocalization of AQP4 and S100β
Immunoreactivity for AQP4 (A, D), S100β (B, E) and merged (C, F) is shown for two representative astrocytes in 3-week-old mouse CA1 SLM. In both panels, colocalization of AQP4 and S100β along the plasma membrane and processes can be observed. In one case (A-C), astrocyte morphology is clearly seen along with surrounding blood vessels. In the other case (D-F), colocalization of AQP4 and S100β is seen also in an astrocyte endfoot contacting a blood vessel. Scale bar, 20 μm.
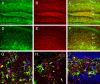
Figure 6. Marginal colocalization of AQP4 and NG2
Double-label immunoreactivity (C, F) for AQP4 (green, A, D) and NG-2 (red, B, E) is seen in DG (A-C) and CA1 (D-F) of the hippocampus. NG2-labeled cells appear throughout the hippocampus but mostly lack co-labeling with AQP4. Confocal images (G-I) from CA1 SR (G), DG ML (H) and DG hilus (I) demonstrate NG2-labeled cells (red) (Nissl, blue) and AQP4-labeled cells (green). SO=stratum oriens; SP= stratum pyramidale; SR=stratum radiatum; SLM=stratum lacunosum moleculare; ML=molecular layer; DGC=dentate granule cell layer; H=hilus. Scale bars: A-F, 100 μm, G-I 20 μm.
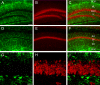
Figure 7. Lack of colocalization of AQP4 and NeuN
Double-label immunoreactivity (C, F) for AQP4 (green, A, D) and NeuN (red, B, E) is seen in DG (A-C) and CA1 (D-F) of the hippocampus. NeuN-labeled cells appear particularly in the principal cell layers throughout the hippocampus but are not co-labeled with AQP4. Confocal series from the DGC (G-I) shows NeuN-labeled granule cells but lack of colocalization of AQP4 and NeuN immunoreactivity. SO=stratum oriens; SP= stratum pyramidale; SR=stratum radiatum; SLM=stratum lacunosum moleculare; ML=molecular layer; DGC=dentate granule cell layer; H=hilus. Scale bars: A-F, 100 μm, G-I 20 μm.
Figure 8. Co-expression of AQP4 and Kir4.1 mRNA by identified hippocampal glial cells
A. A freshly isolated astrocyte was de- and hyperpolarized between +30 mV and -120 mV (10 mV increments; holding potential -60 mV; left). Then, the same protocol was applied after adding 100 μM BaCl2 to the bath (middle); the right panel shows the Ba2+-sensitive current. B. I/V-relation of the Ba2+-sensitive currents (left). Note that currents reversed close to the K+ equilibrium potential (-82.3 mV). DNA gels demonstrate that the recorded cell co-expressed Kir4.1 and AQP4 transcripts (right). C. An NG2 cell was analyzed as described above (holding potential -70 mV; de- and hyperpolarization between +20 and -130 mV) (left). The cell also co-expressed AQP4 and Kir4.1 mRNA (right).
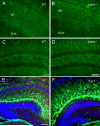
Figure 9. Deletion of AQP4 or Kir4.1 does not dramatically affect Kir4.1 or AQP4 immunoreactivity
Kir4.1 immunoreactivity in adult hippocampus in WT (A, C) and AQP4-/- mice (B, D). Note the abundance of astrocytic process immunoreactivity in both CA1 (A, B) and dentate gyrus (C, D) in both genotypes. Apparently, CA1 stratum radiatum (sr) displays more intense immunoreactivity than CA1 stratum lacunosum moleculare (slm) in both genotypes (A, B). Scale bar, 100 μm. AQP4 immunoreactivity in P9 hippocampus in WT (E) and Kir4.1-/- (F) mice. A similar pattern of AQP4 immunoreactivity is observed with blood vessel and parenchymal immunoreactivity indicating no dramatic effects of Kir4.1 deletion on AQP4 immunoreactivity. Scale bar, 200 μm.
Similar articles
- Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells.
Zhang H, Verkman AS. Zhang H, et al. Mol Cell Neurosci. 2008 Jan;37(1):1-10. doi: 10.1016/j.mcn.2007.08.007. Epub 2007 Aug 15. Mol Cell Neurosci. 2008. PMID: 17869537 Free PMC article. - Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis.
Lee DJ, Hsu MS, Seldin MM, Arellano JL, Binder DK. Lee DJ, et al. Exp Neurol. 2012 May;235(1):246-55. doi: 10.1016/j.expneurol.2012.02.002. Epub 2012 Feb 14. Exp Neurol. 2012. PMID: 22361023 Free PMC article. - Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus.
Strohschein S, Hüttmann K, Gabriel S, Binder DK, Heinemann U, Steinhäuser C. Strohschein S, et al. Glia. 2011 Jun;59(6):973-80. doi: 10.1002/glia.21169. Epub 2011 Mar 28. Glia. 2011. PMID: 21446052 - Astroglial Kir4.1 and AQP4 Channels: Key Regulators of Potassium Homeostasis and Their Implications in Autism Spectrum Disorders.
Abbasian V, Davoudi S, Vahabzadeh A, Maftoon-Azad MJ, Janahmadi M. Abbasian V, et al. Cell Mol Neurobiol. 2025 Jun 11;45(1):56. doi: 10.1007/s10571-025-01574-w. Cell Mol Neurobiol. 2025. PMID: 40498219 Free PMC article. Review. - Astrogliogenesis in human fetal brain: complex spatiotemporal immunoreactivity patterns of GFAP, S100, AQP4 and YKL-40.
Holst CB, Brøchner CB, Vitting-Seerup K, Møllgård K. Holst CB, et al. J Anat. 2019 Sep;235(3):590-615. doi: 10.1111/joa.12948. Epub 2019 Mar 22. J Anat. 2019. PMID: 30901080 Free PMC article. Review.
Cited by
- Advances in the Potential Biomarkers of Epilepsy.
Kobylarek D, Iwanowski P, Lewandowska Z, Limphaibool N, Szafranek S, Labrzycka A, Kozubski W. Kobylarek D, et al. Front Neurol. 2019 Jul 2;10:685. doi: 10.3389/fneur.2019.00685. eCollection 2019. Front Neurol. 2019. PMID: 31312171 Free PMC article. Review. - Expression of the Astrocyte Water Channel Aquaporin-4 in the Mouse Brain.
Hubbard JA, Hsu MS, Seldin MM, Binder DK. Hubbard JA, et al. ASN Neuro. 2015 Oct 21;7(5):1759091415605486. doi: 10.1177/1759091415605486. Print 2015 Sep-Oct. ASN Neuro. 2015. PMID: 26489685 Free PMC article. - Insights into Cell Surface Expression, Supramolecular Organization, and Functions of Aquaporin 4 Isoforms in Astrocytes.
Jorgačevski J, Zorec R, Potokar M. Jorgačevski J, et al. Cells. 2020 Dec 7;9(12):2622. doi: 10.3390/cells9122622. Cells. 2020. PMID: 33297299 Free PMC article. Review. - The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy.
Owasil R, O'Neill R, Keable A, Nimmo J, MacGregor Sharp M, Kelly L, Saito S, Simpson JE, Weller RO, Smith C, Attems J, Wharton SB, Yuen HM, Carare RO. Owasil R, et al. Int J Mol Sci. 2020 Feb 12;21(4):1225. doi: 10.3390/ijms21041225. Int J Mol Sci. 2020. PMID: 32059400 Free PMC article. - From Homeostasis to Pathology: Decoding the Multifaceted Impact of Aquaporins in the Central Nervous System.
Toader C, Tataru CP, Florian IA, Covache-Busuioc RA, Dumitrascu DI, Glavan LA, Costin HP, Bratu BG, Ciurea AV. Toader C, et al. Int J Mol Sci. 2023 Sep 20;24(18):14340. doi: 10.3390/ijms241814340. Int J Mol Sci. 2023. PMID: 37762642 Free PMC article. Review.
References
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. - PubMed
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100:13615–13620. - PMC - PubMed
- Andrew RD, Fagan M, Ballyk BA, Rosen AS. Seizure susceptibility and the osmotic state. Brain Res. 1989;498:175–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous