Mouse models of advanced spontaneous metastasis for experimental therapeutics - PubMed (original) (raw)
Review
Mouse models of advanced spontaneous metastasis for experimental therapeutics
Giulio Francia et al. Nat Rev Cancer. 2011 Feb.
Abstract
An enduring problem in cancer research is the failure to reproduce highly encouraging preclinical therapeutic findings using transplanted or spontaneous primary tumours in mice in clinical trials of patients with advanced metastatic disease. There are several reasons for this, including the failure to model established, visceral metastatic disease. We therefore developed various models of aggressive multi-organ spontaneous metastasis after surgical resection of orthotopically transplanted human tumour xenografts. In this Opinion article we provide a personal perspective summarizing the prospect of their increased clinical relevance. This includes the reduced efficacy of certain targeted anticancer drugs, the late emergence of spontaneous brain metastases and the clinical trial results evaluating a highly effective therapeutic strategy previously tested using such models.
Figures
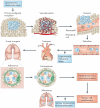
Figure 1. The metastatic cascade
Metastatically competent primary tumours grow, invade the local host tissue and eventually shed tumour cells into the circulation. These cells travel to and colonize distant organs, and their subsequent growth at the secondary sites constitutes metastatic disease,. Spontaneous metastasis assays involve establishing a primary tumour that is allowed to grow and spread in the host, whereas experimental metastasis assays circumvent the initial growth and invasion stages as a result of directly injecting tumour cells into the circulation. Other assays mimic aspects of metastatic growth by seeding tumour cells at the secondary site (for example, by intrasplenic injection of colorectal cancer cells, which directly targets the cells to the liver where they grow as metastases). Figure is modified, with permission, from REF. © (1982) Amerian Association for the Advancement of Science.
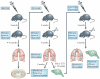
Figure 2. The selection of metastatically aggressive subpopulations of tumour cells often requires rounds of in vivo selection
For example, a primary tumour of the human melanoma cell line WM239 was established by the injection of the cells subdermally (orthotopically) into severe combined immunodeficient (SCID) mice, allowed to grow and establish itself for 3 weeks, and was then surgically removed (to prevent the rapidly growing primary tumour from causing end point termination of the experiment). Several months later the mice showed evidence of lung metastases — from which the subline 113/6-4L was derived. In some studies, these cells were re-implanted into a new host for an additional round of selection. This type of in vivo enrichment procedure was also used to derive, for example, the highly metastatic 231/LM2-4 variant of the human breast cancer cell line MDA-MB-231 (REF. 34). By using a similar selection procedure, the H2N/met2.hCG metastatic variant was derived from MDA-MB-231 cells that previously expressed ERBB2 by gene transduction and which were then transfected to express and secrete the β-subunit of human choriogonadotropin (β-hCG) protein, which can be used as a surrogate molecular marker of disease burden and response to therapy,. The treatment of mice with established 113/6-4L spontaneous metastases using a metronomic doublet vinblastine (Vbl) and CTX regimen led to prolonged survival and to the eventual emergence of brain metastases. Isolation of a brain metastases resulted in the derivation of a variant subpopulation that spontaneously metastasizes to the brain without any therapeutic intervention to prolong the survival times of mice. Figure is modified, with permission, from REF. © (2008) American Association for Cancer Research.
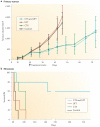
Figure 3. Differential response of metastases and primary tumours to therapy
A disparity between primary tumours (part a) and metastasis (part b) in terms of response to therapy was observed with the human breast cancer model 231/LM2-4 treated with the combination of metronomic daily cyclophosphamide (CTX) and the 5-Fluorouracil (5-FU) oral prodrug UFT (tegafur plus uracil). Thus, primary tumour growth assays involving treatment showed no antitumour effect by combining UFT with CTX, and did not foreshadow the UFT and CTX doublet metronomic therapy (part a). Figure is modified, with permission, from REF. © (2006) American Association for Cancer Research.
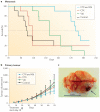
Figure 4. Treatment of visceral metastatic disease can result in the appearance of metastases to the brain
a | Mice with established spontaneous metastases from the highly metastatic variant 113/6-4L derived from the WM239 melanoma were treated using a metronomic doublet vinblastine (Vbl) and cyclophosphamide (CTX) regimen (FIG. 2), and this led to prolonged survival and to the eventual emergence of the brain metastases. b | A therapeutic benefit was not observed when the Vbl and CTX regimen was administered to 113/6-4L grown as primary tumours. c | Image of brain metastasis from this experiment. Figure is modified, with permission, from REF. © (2008) American Association for Cancer Research.
Similar articles
- On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing.
Man S, Munoz R, Kerbel RS. Man S, et al. Cancer Metastasis Rev. 2007 Dec;26(3-4):737-47. doi: 10.1007/s10555-007-9087-6. Cancer Metastasis Rev. 2007. PMID: 17846863 Review. - Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic.
Hoffman RM. Hoffman RM. Invest New Drugs. 1999;17(4):343-59. doi: 10.1023/a:1006326203858. Invest New Drugs. 1999. PMID: 10759402 Review. - Development of Patient Derived Xenograft Models of Overt Spontaneous Breast Cancer Metastasis: A Cautionary Note.
Paez-Ribes M, Man S, Xu P, Kerbel RS. Paez-Ribes M, et al. PLoS One. 2016 Jun 29;11(6):e0158034. doi: 10.1371/journal.pone.0158034. eCollection 2016. PLoS One. 2016. PMID: 27355476 Free PMC article.
Cited by
- Near infrared photoimmunotherapy for lung metastases.
Sato K, Nagaya T, Mitsunaga M, Choyke PL, Kobayashi H. Sato K, et al. Cancer Lett. 2015 Aug 28;365(1):112-21. doi: 10.1016/j.canlet.2015.05.018. Epub 2015 May 27. Cancer Lett. 2015. PMID: 26021765 Free PMC article. - The role of tumour-stromal interactions in modifying drug response: challenges and opportunities.
McMillin DW, Negri JM, Mitsiades CS. McMillin DW, et al. Nat Rev Drug Discov. 2013 Mar;12(3):217-28. doi: 10.1038/nrd3870. Nat Rev Drug Discov. 2013. PMID: 23449307 Review. - CAM-Delam: an in vivo approach to visualize and quantify the delamination and invasion capacity of human cancer cells.
Palaniappan TK, Šlekienė L, Jonasson AK, Gilthorpe J, Gunhaga L. Palaniappan TK, et al. Sci Rep. 2020 Jun 26;10(1):10472. doi: 10.1038/s41598-020-67492-7. Sci Rep. 2020. PMID: 32591581 Free PMC article. - Nanotechnology: Future of Oncotherapy.
Gharpure KM, Wu SY, Li C, Lopez-Berestein G, Sood AK. Gharpure KM, et al. Clin Cancer Res. 2015 Jul 15;21(14):3121-30. doi: 10.1158/1078-0432.CCR-14-1189. Clin Cancer Res. 2015. PMID: 26180057 Free PMC article. Review. - Impact of limb amputation and cisplatin chemotherapy on metastatic progression in mouse models of osteosarcoma.
Ren L, Huang S, Beck J, LeBlanc AK. Ren L, et al. Sci Rep. 2021 Dec 24;11(1):24435. doi: 10.1038/s41598-021-04018-9. Sci Rep. 2021. PMID: 34952927 Free PMC article.
References
- Wilmanns C, Fan D, O’Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int. J. Cancer. 1992;52:98–104. - PubMed
- Kerbel RS. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 1998;17:301–304. - PubMed
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived - but they can. be improved. Cancer Biol. Ther. 2003;2:108–113. - PubMed
- Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer. 2004;40:837–844. - PubMed
- Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases