Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats - PubMed (original) (raw)
Randomized Controlled Trial
Affiliations
- PMID: 21262119
- PMCID: PMC2958202
Randomized Controlled Trial
Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats
Robert T Dauchy et al. Comp Med. 2010 Oct.
Abstract
Dark-phase light contamination can significantly disrupt chronobiologic rhythms, thereby potentially altering the endocrine physiology and metabolism of experimental animals and influencing the outcome of scientific investigations. We sought to determine whether exposure to low-level light contamination during the dark phase influenced the normally entrained circadian rhythms of various substances in plasma. Male Sprague-Dawley rats (n = 6 per group) were housed in photobiologic light-exposure chambers configured to create 1) a 12:12-h light:dark cycle without dark-phase light contamination (control condition; 123 μW/cm(2), lights on at 0600), 2) experimental exposure to a low level of light during the 12-h dark phase (with 0.02, 0.05, 0.06, or 0.08 μW/cm(2) light at night), or 3) constant bright light (123 μW/cm(2)). Dietary and water intakes were recorded daily. After 2 wk, rats underwent 6 low-volume blood draws at 4-h intervals (beginning at 0400) during both the light and dark phases. Circadian rhythms in dietary and water intake and levels of plasma total fatty acids and lipid fractions remained entrained during exposure to either control conditions or low-intensity light during the dark phase. However, these patterns were disrupted in rats exposed to constant bright light. Circadian patterns of plasma melatonin, glucose, lactic acid, and corticosterone were maintained in all rats except those exposed to constant bright light or the highest level of light during the dark phase. Therefore even minimal light contamination during the dark phase can disrupt normal circadian rhythms of endocrine metabolism and physiology and may alter the outcome of scientific investigations.
Figures
Figure 1.
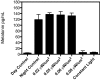
sPlasma melatonin levels (pg/mL; mean ± 1 SD) of Sprague–Dawley rats (n = 6 per group) maintained for 6 wk on either a control 12:12-h light:dark cycle (300 lx; 123 μW/cm2; lights on, 0600; group 1); experimental light-at-night (LAN) lighting cycles (that is, a 12:12-h light:dark cycle with light contamination during the dark phase) with 0.02 (group 2); 0.04 (group 3), 0.06 (group 4), 0.08 (group 5) μW/cm2 during the dark phase; or constant bright light (group 6). Plasma samples for controls were obtained at either daytime (1600, day control) or nighttime (0400, night control) and for experimental groups (2 through 6) during the dark phase at 0400. Melatonin levels in the control group at 0400 and 1600 differed significantly (P < 0.05); melatonin levels for groups 1 (1600), 5 (0400), and 6 (0400) did not differ between each other or with those of groups 2 through 4 (0400).
Figure 2.
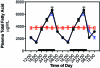
Diurnal changes in plasma total fatty acids in the arterial blood of animals in groups 1 through 4 (closed circles), 5 (triangles), and constant bright light (open circles, group 6) fed normal chow ad libitum. Animals were subjected to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600. Values are expressed as μg/mL plasma, and total fatty acid values are the sums of myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acids. Each point represents mean ± 1 SD (n = 6 per group). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
Figure 3.
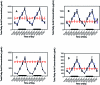
Diurnal changes in the blood plasma lipid concentrations in the arterial blood of adult male Sprague–Dawley rats fed normal chow ad libitum. Animals were subjected to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600 (indicated by dark bars). Total fatty acid values (μg/mL plasma; mean ± 1 SD; n = 6 per group) for groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles) were the sums of myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acids in the different lipid classes of (A) triglycerides, (B) free fatty acids, (C) phospholipids, and (D) cholesterol esters collected at the various time points. Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
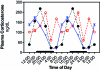
Figure 4.
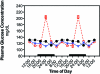
Diurnal changes in plasma glucose levels (mg/dL; mean ± 1 SD; n = 6 per group) in the arterial blood of rats in groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles). Rats were exposed to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600 (dark bars). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
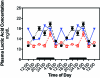
Figure 5.
Diurnal changes in plasma lactic acid concentrations (mg/dL; mean ± 1 SD; n = 6 per group) in the arterial blood of rats in groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles). Rats were exposed to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600 (dark bars). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
Figure 6.
Diurnal changes in plasma corticosterone concentrations (ng/dL; mean ± 1 SD; n = 6 per group) in the arterial blood of rats in groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles). Rats were exposed to dark-phase cycles (as described in the legend to Figure 1) from 1800 to 0600 (dark bars). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
Similar articles
- The influence of red light exposure at night on circadian metabolism and physiology in Sprague-Dawley rats.
Dauchy RT, Wren MA, Dauchy EM, Hoffman AE, Hanifin JP, Warfield B, Jablonski MR, Brainard GC, Hill SM, Mao L, Dobek GL, Dupepe LM, Blask DE. Dauchy RT, et al. J Am Assoc Lab Anim Sci. 2015 Jan;54(1):40-50. J Am Assoc Lab Anim Sci. 2015. PMID: 25651090 Free PMC article. - Effect of Isoflurane Anesthesia on Circadian Metabolism and Physiology in Rats.
Wren-Dail MA, Dauchy RT, Blask DE, Hill SM, Ooms TG, Dupepe LM, Bohm RP Jr. Wren-Dail MA, et al. Comp Med. 2017 Mar 1;67(2):138-146. Comp Med. 2017. PMID: 28381314 Free PMC article. - Effect of different spectral transmittances through tinted animal cages on circadian metabolism and physiology in Sprague-Dawley rats.
Wren MA, Dauchy RT, Hanifin JP, Jablonski MR, Warfield B, Brainard GC, Blask DE, Hill SM, Ooms TG, Bohm RP Jr. Wren MA, et al. J Am Assoc Lab Anim Sci. 2014 Jan;53(1):44-51. J Am Assoc Lab Anim Sci. 2014. PMID: 24411779 Free PMC article. - Differential Effects of Constant Light and Dim Light at Night on the Circadian Control of Metabolism and Behavior.
Rumanova VS, Okuliarova M, Zeman M. Rumanova VS, et al. Int J Mol Sci. 2020 Jul 31;21(15):5478. doi: 10.3390/ijms21155478. Int J Mol Sci. 2020. PMID: 32751870 Free PMC article. Review. - Melatonin and the circadian system: contributions to successful female reproduction.
Reiter RJ, Tamura H, Tan DX, Xu XY. Reiter RJ, et al. Fertil Steril. 2014 Aug;102(2):321-8. doi: 10.1016/j.fertnstert.2014.06.014. Epub 2014 Jul 1. Fertil Steril. 2014. PMID: 24996495 Review.
Cited by
- A Method for Growing Tissue-Isolated Human Tumor Xenografts in Nude Rats for Melatonin/Cancer Studies.
Dauchy RT, Hill SM, Blask DE. Dauchy RT, et al. Methods Mol Biol. 2022;2550:489-496. doi: 10.1007/978-1-0716-2593-4_47. Methods Mol Biol. 2022. PMID: 36180716 - Evaluating chronotypically tailored light therapy for breast cancer survivors: Preliminary findings on fatigue and disrupted sleep.
Wu HS, Gao F, Yan L, Given C. Wu HS, et al. Chronobiol Int. 2022 Feb;39(2):221-232. doi: 10.1080/07420528.2021.1992419. Epub 2021 Nov 3. Chronobiol Int. 2022. PMID: 34732099 Free PMC article. Clinical Trial. - Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds.
Dominoni DM, Goymann W, Helm B, Partecke J. Dominoni DM, et al. Front Zool. 2013 Oct 3;10(1):60. doi: 10.1186/1742-9994-10-60. Front Zool. 2013. PMID: 24090446 Free PMC article. - Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer.
Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, Blask DE, Hill SM. Dauchy RT, et al. Cancer Res. 2014 Aug 1;74(15):4099-110. doi: 10.1158/0008-5472.CAN-13-3156. Cancer Res. 2014. PMID: 25062775 Free PMC article. - Dim light at night disturbs the daily sleep-wake cycle in the rat.
Stenvers DJ, van Dorp R, Foppen E, Mendoza J, Opperhuizen AL, Fliers E, Bisschop PH, Meijer JH, Kalsbeek A, Deboer T. Stenvers DJ, et al. Sci Rep. 2016 Oct 20;6:35662. doi: 10.1038/srep35662. Sci Rep. 2016. PMID: 27762290 Free PMC article.
References
- Acuna-Castroviejo D, Reiter RJ, Menedez-Pelaez A, Pablos MI, Burgos A. 1994. Characterization of high-affinity melatonin binding sites in the purified cell nuclei of rat liver. J Pineal Res 16:100–112 - PubMed
- Aschoff J. 1960. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25:11–28 - PubMed
- Aschoff J. 1981. Handbook of behavioral neurobiology, biological rhythms, p 1–581 New York (NY): Plenum Press
- Bailey CJ, Atkins TW, Matty AJ. 1974. Melatonin inhibition of insulin secretion in the rat and mouse. Horm Res 5:21–28 - PubMed
- Bellhorn RW. 1980. Lighting in the animal environment. Lab Anim Sci 30:440–450 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources